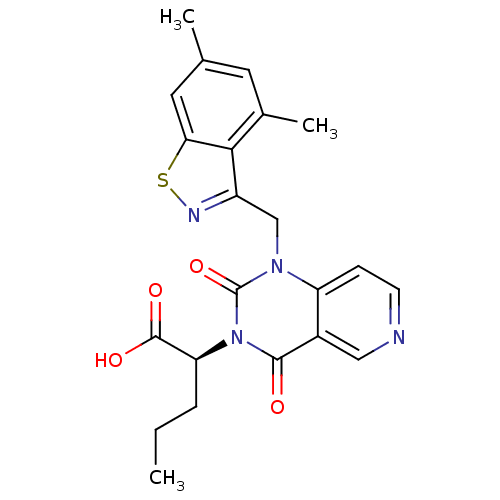
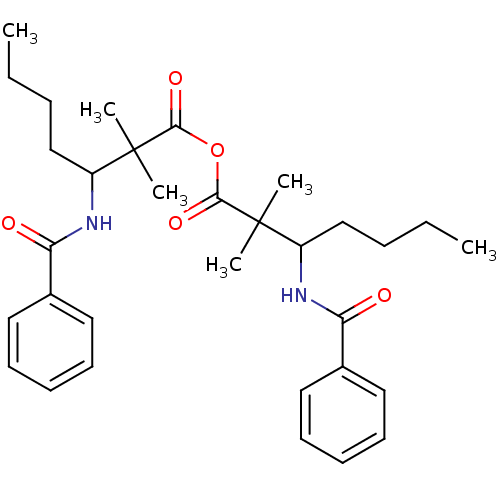
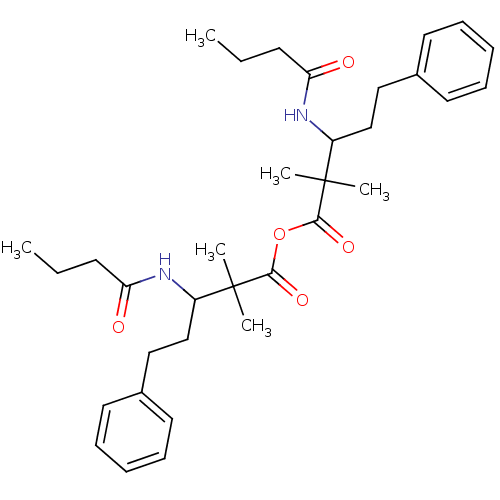
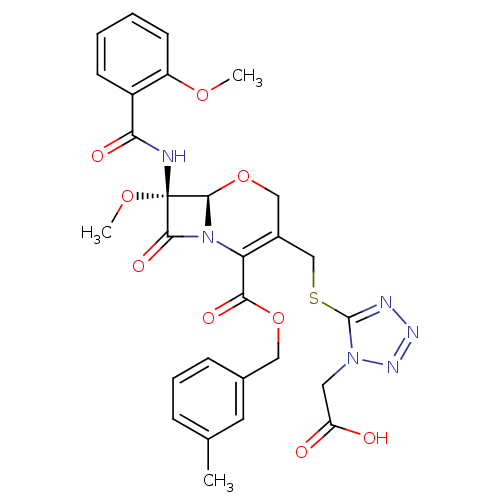
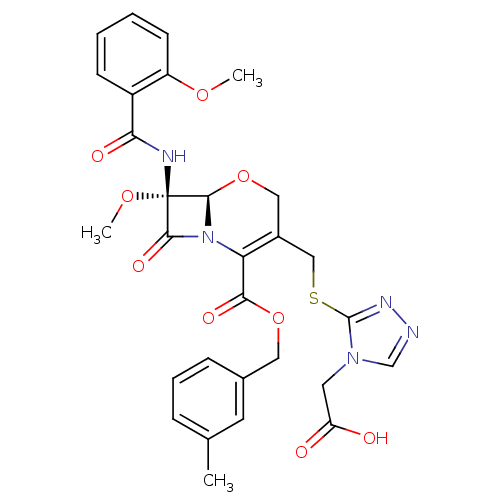
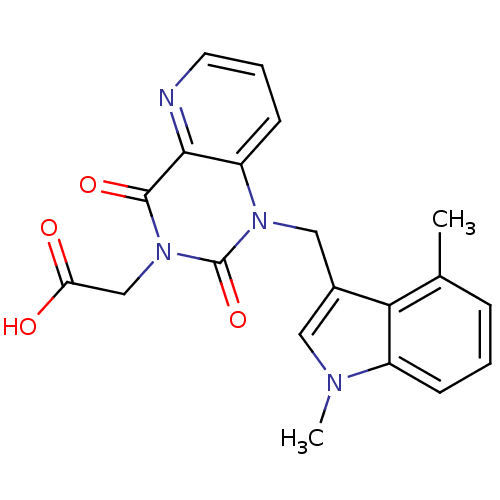
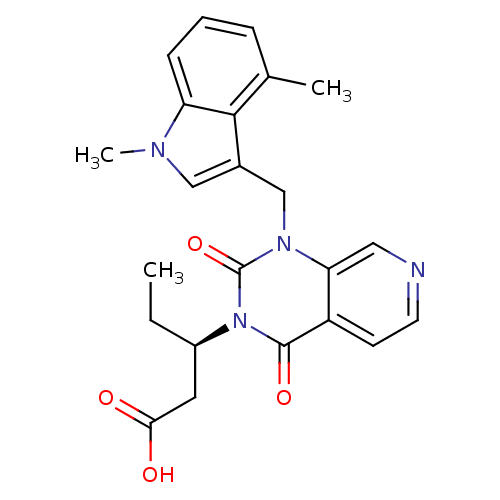
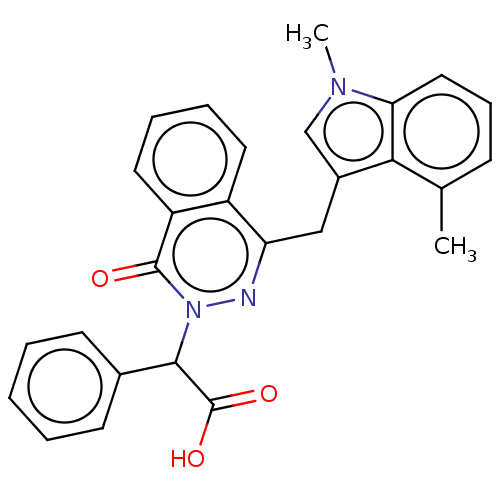
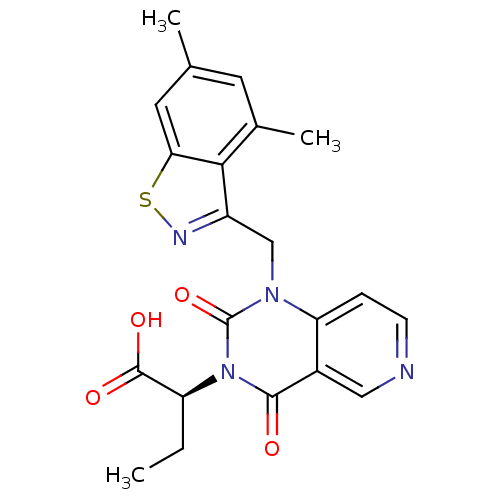
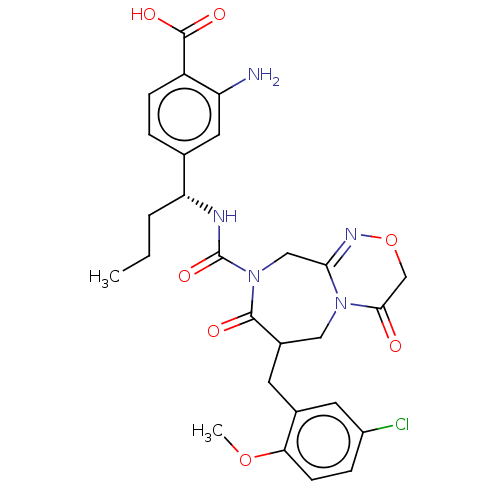
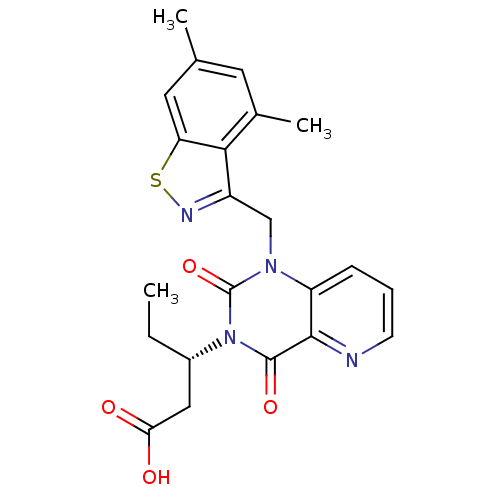
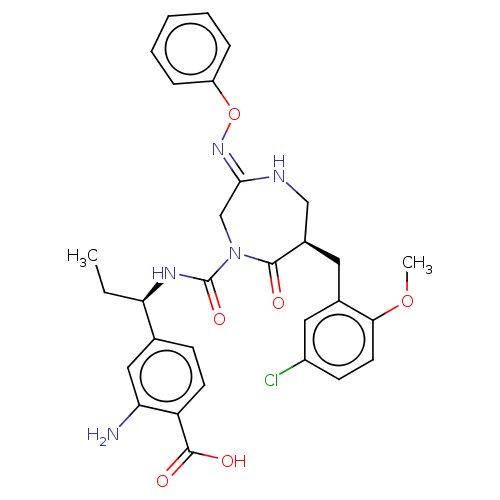
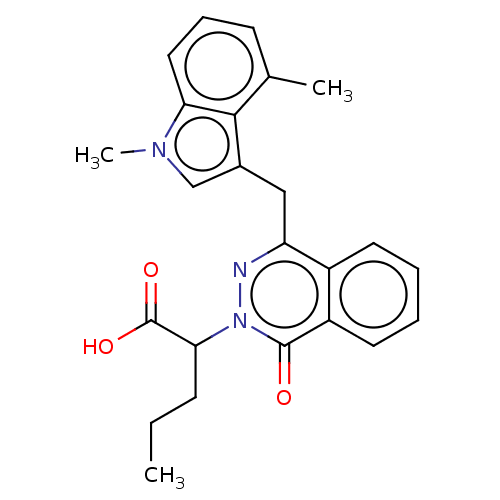
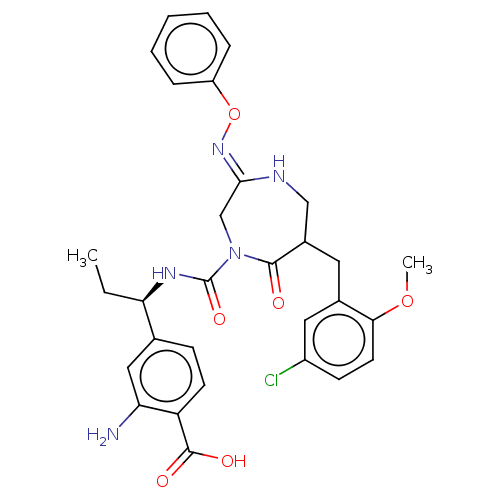
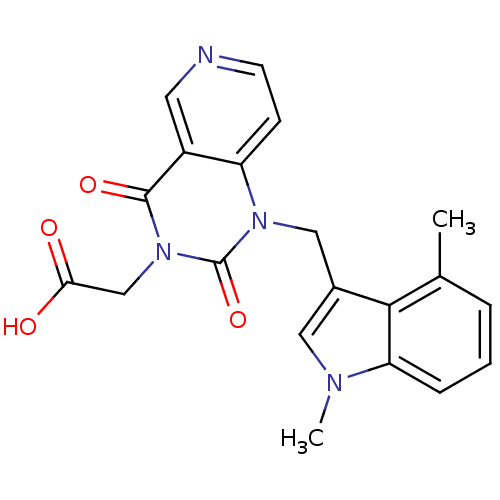
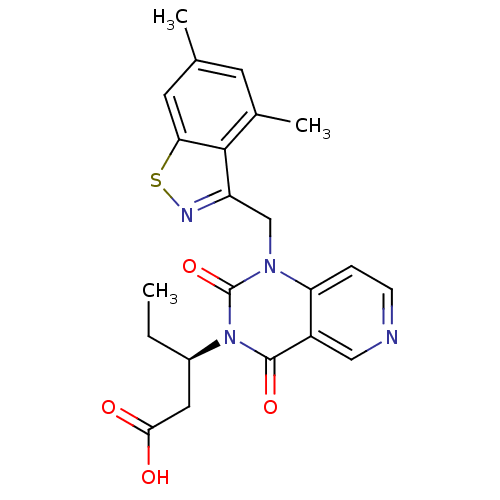
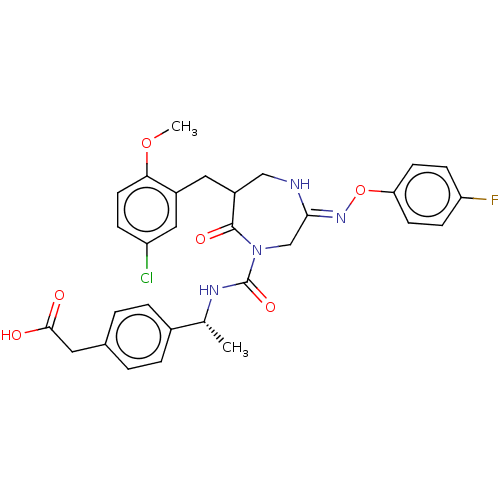
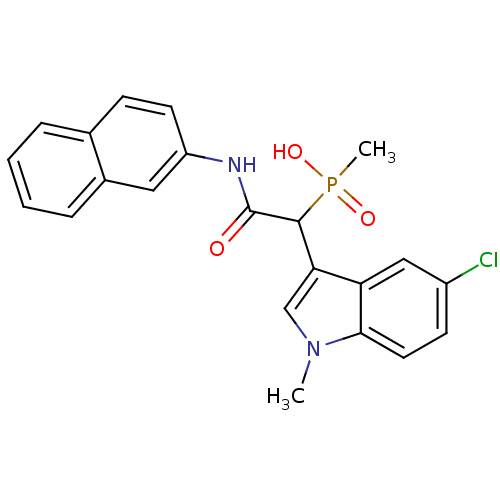
Affinity DataIC50: 0.300nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of human ChymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Compound was evaluated for its inhibitory activity against human Serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Compound was evaluated for its inhibitory activity against human Serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Compound was evaluated for its inhibitory activity against human Serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human ChymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Compound was evaluated for its inhibitory activity against human Serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against human chymase (h-chymase)More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibitory activity against bovine pancreatic alpha-chymotrypsin (alpha-CT)More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibitory activity against human chymase (h-chymase)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human Serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibitory activity against human serine protease chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 8.0Assay Description:Chymase assays were performed in a total volume of 15 uL in Corning black opaque 384-well microtiter plates with a non-binding surface (Corning, N.Y....More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 8.0Assay Description:Chymase assays were performed in a total volume of 15 uL in Corning black opaque 384-well microtiter plates with a non-binding surface (Corning, N.Y....More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 9.40nMAssay Description:Inhibition of chymase (unknown origin) by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.60nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMpH: 8.0 T: 2°CAssay Description:In vitro inhibition assay of Chymase.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used fo...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair

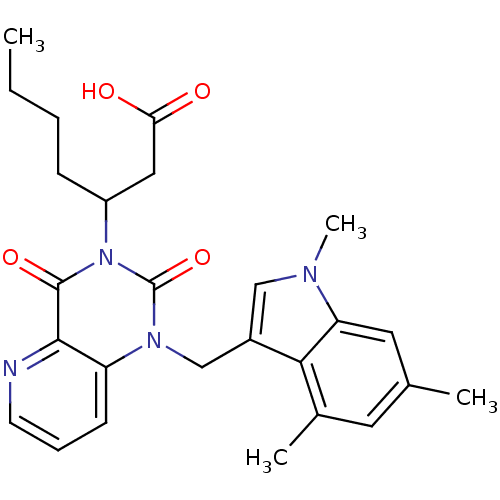
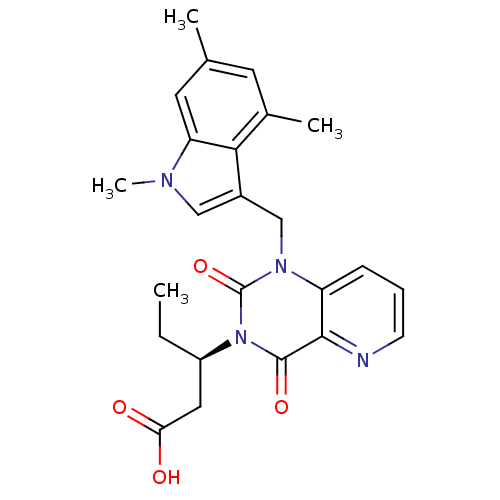



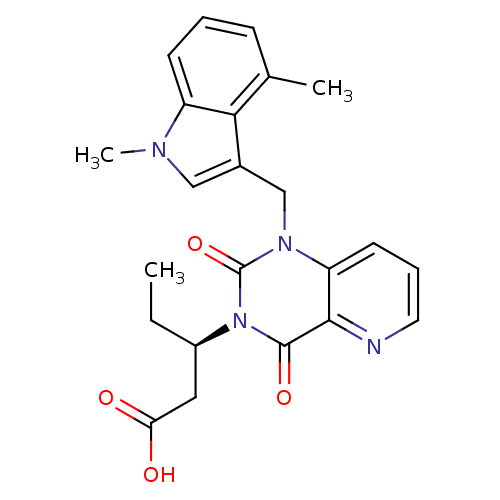
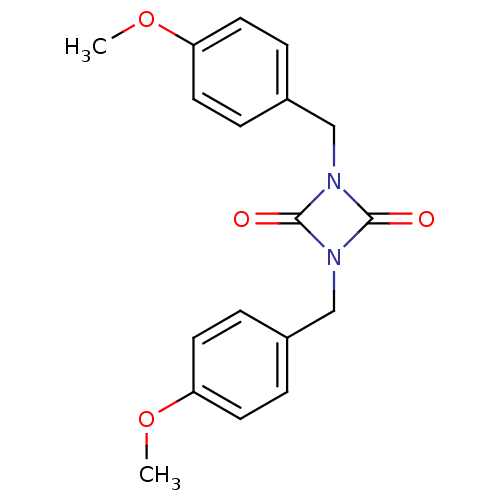
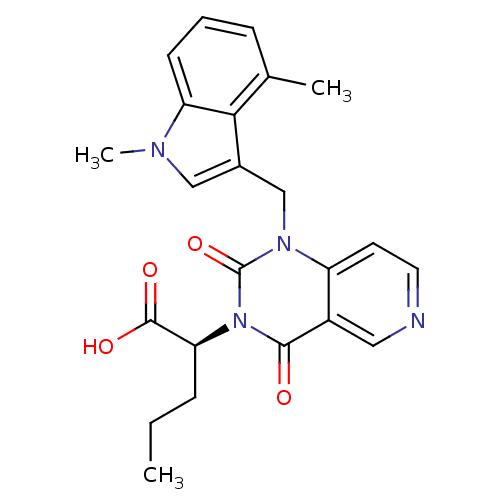
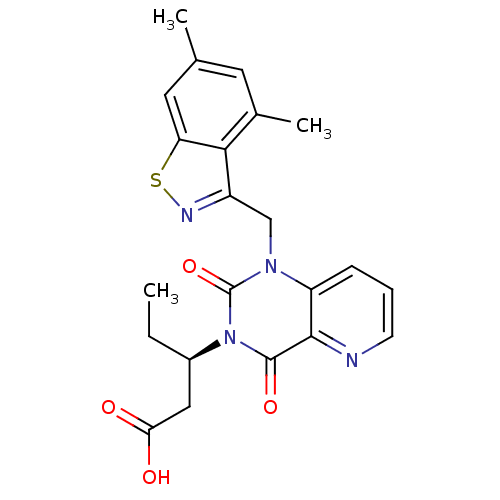
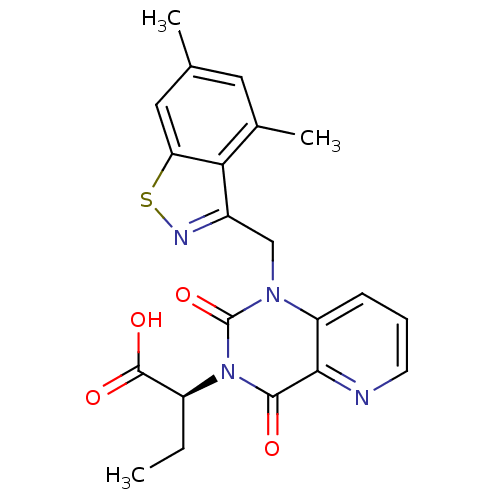
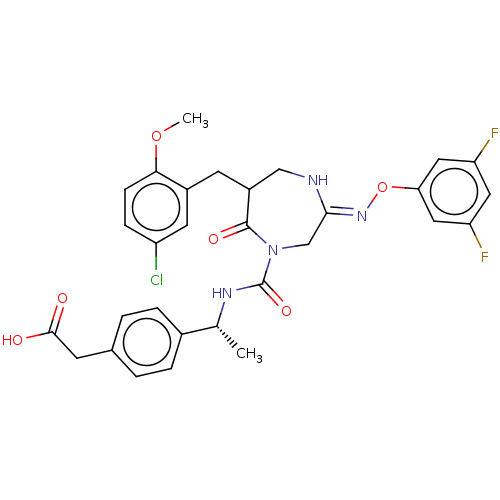
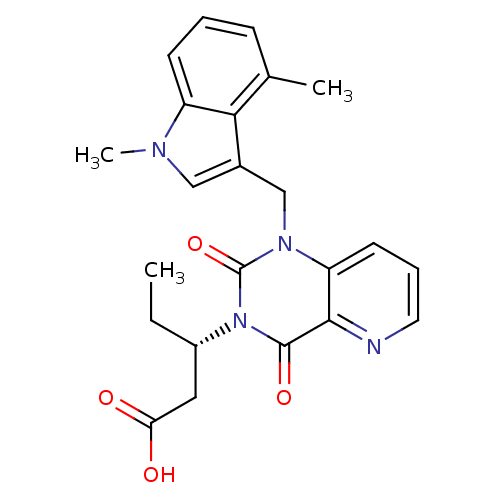
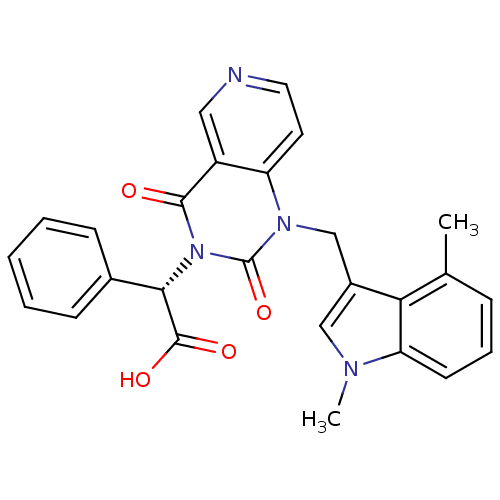
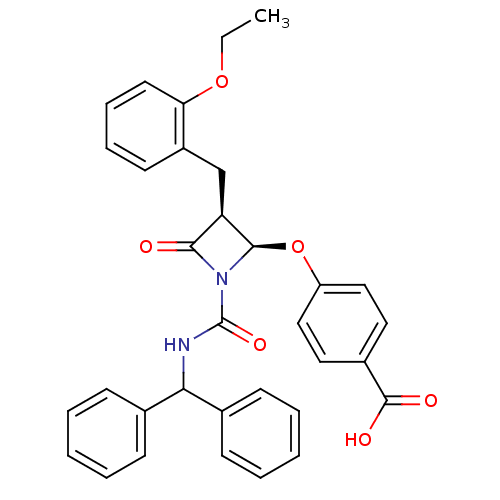
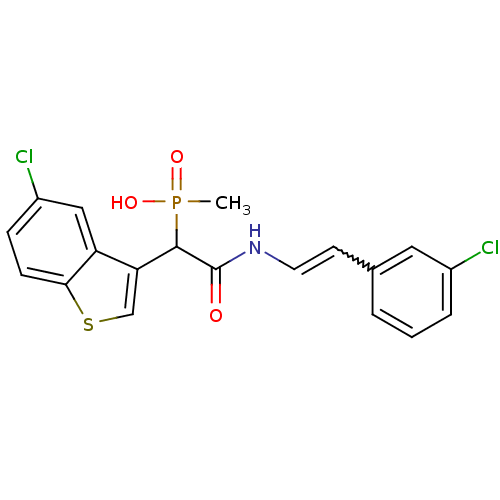
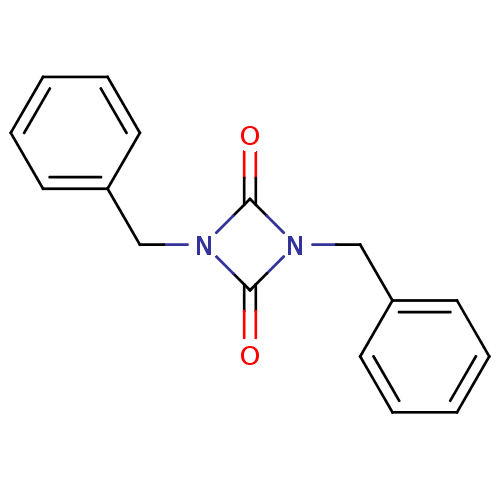
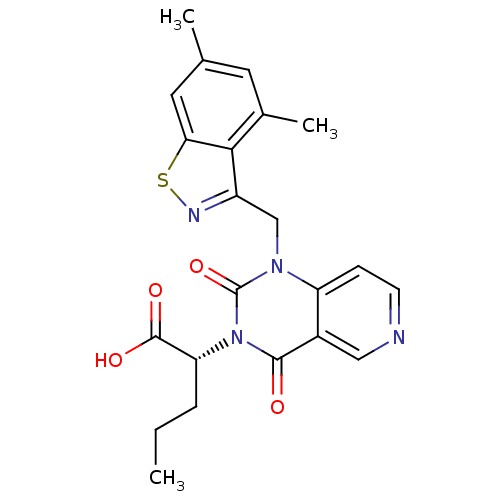
 3D Structure (crystal)
3D Structure (crystal)