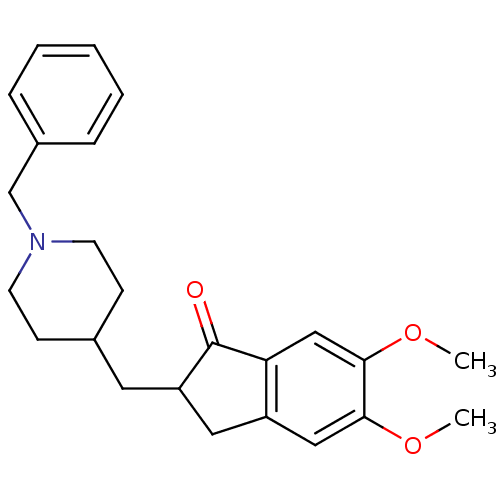
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 5.20nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 6.70nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 27nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 260nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 310nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 350nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 420nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 460nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 800nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 990nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.10E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 2.10E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 2.20E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 5.20E+3nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 6.70E+3nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 7.40E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 7.60E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.05E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.81E+4nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 3.05E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 4.03E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 4.31E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 6.54E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 8.22E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: >1.00E+5nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: >1.00E+5nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.30E+5nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.43E+5nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 5.00E+5nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 7.45E+5nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.46E+6nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.64E+6nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 2.77E+6nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.13E+7nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 1.54E+7nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 8.55E+8nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)