Affinity DataKi: 270nM ΔG°: -38.1kJ/molepH: 7.0 T: 2°CAssay Description:A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nM ΔG°: -33.0kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing...More data for this Ligand-Target Pair
Affinity DataKi: 8.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant AKR1C3-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
Affinity DataIC50: 730nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of AKR1C3 using 9,10-phenanthroquinone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human recombinant AKR1C3 expressed in Escherichia coli JM109 cells using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 3.65E+4nMAssay Description:Inhibition of human recombinant AKR1C2-mediated NADP+-dependent oxidation of S-(+)-1,2,3,4-tetrahydro-1-naphtholMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of human recombinant AKR1C2 expressed in Escherichia coli using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C2 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of human recombinant GST-tagged AKR1C1 expressed in Escherichia coli using S-tetralol as substrate by fluorometryMore data for this Ligand-Target Pair

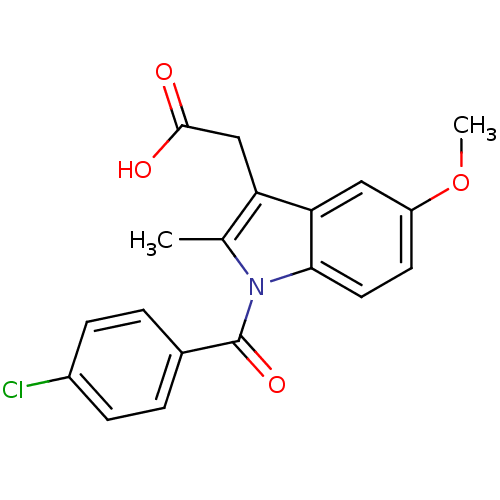
 3D Structure (crystal)
3D Structure (crystal)