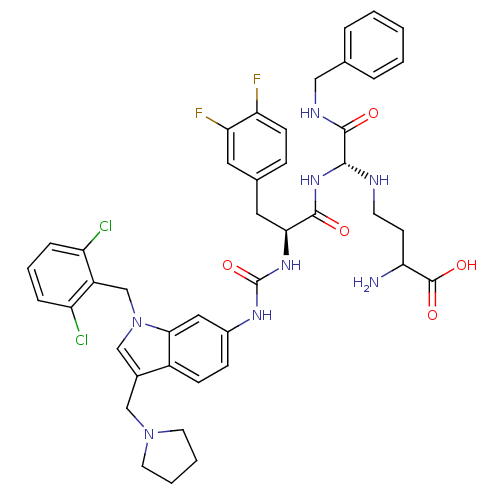
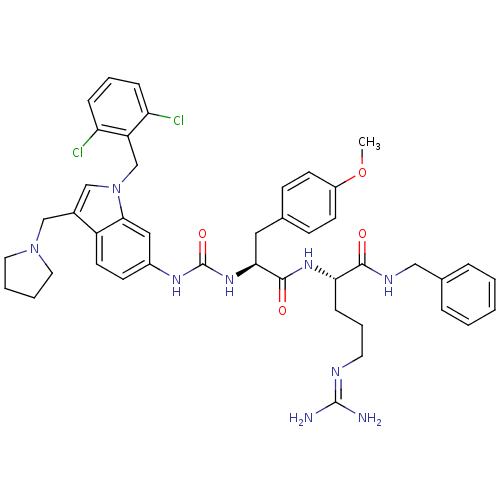
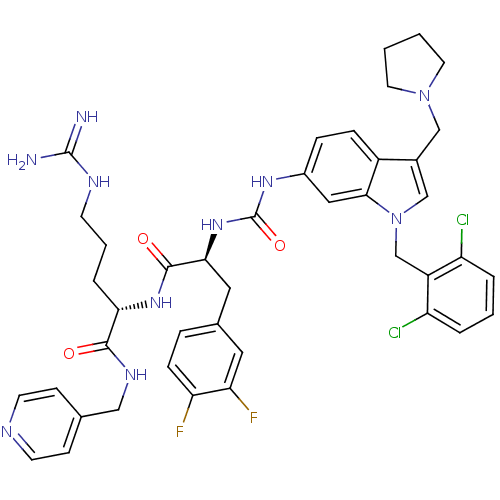
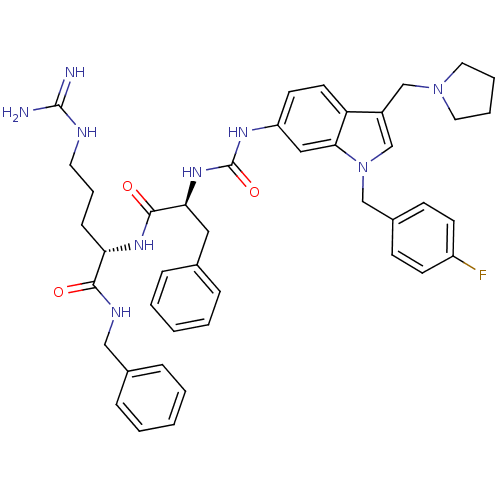
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 570nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.06E+3nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.32E+3nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.33E+4nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.78E+4nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.82E+4nMAssay Description:In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM.More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 2.20nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.820nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.0400nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.150nMAssay Description:Binding Affinity of ligand against Protease-activated Receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.700nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 2.40nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 10.7nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 1.30nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKd: 0.440nMAssay Description:Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM)More data for this Ligand-Target Pair