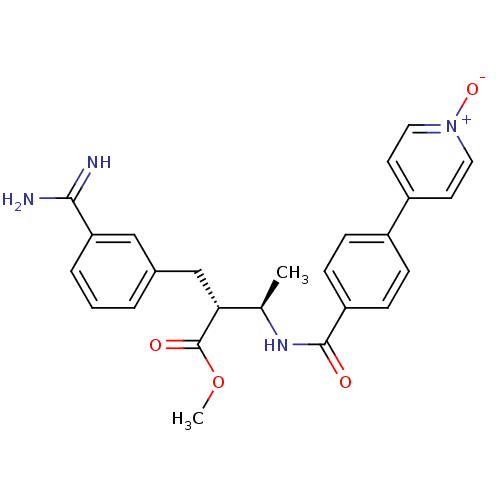
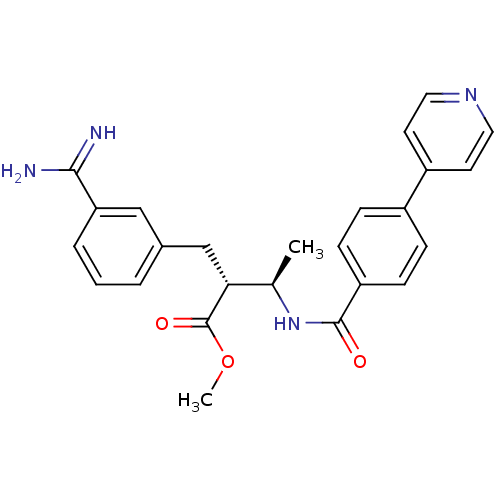
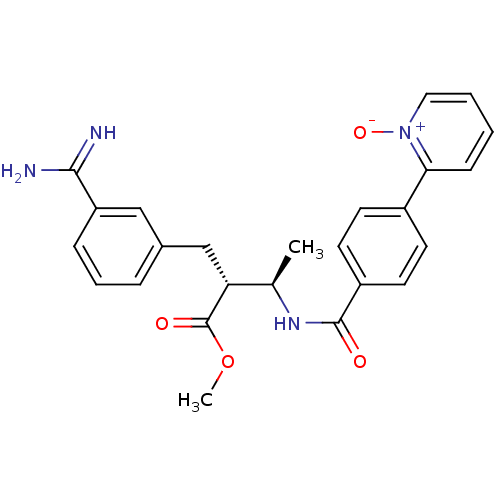
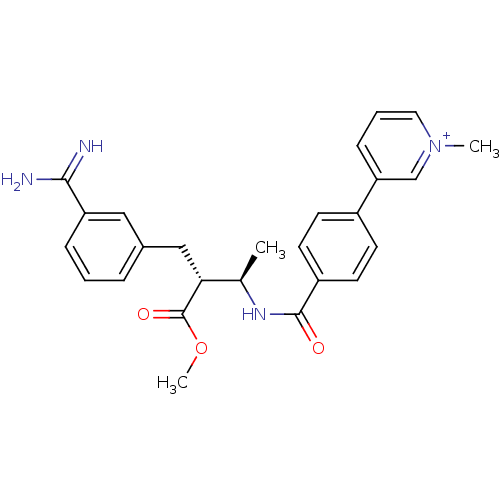
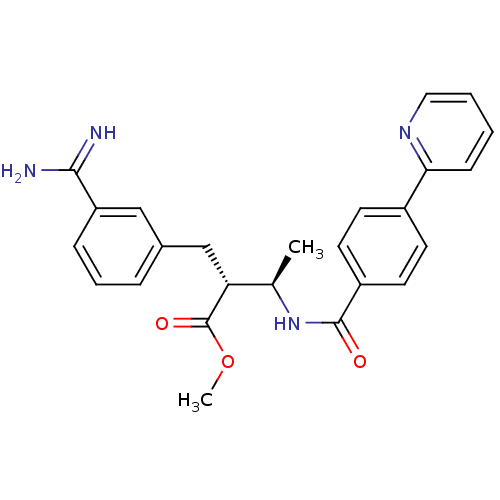
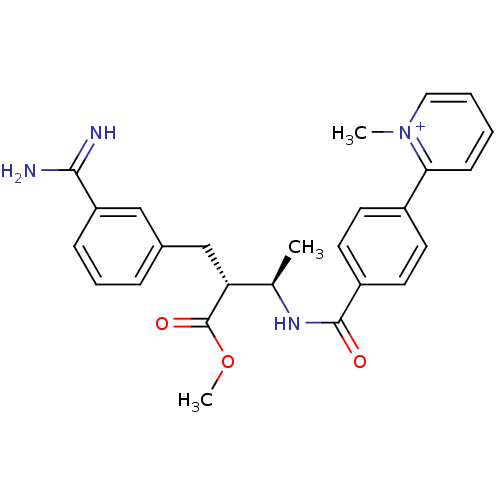
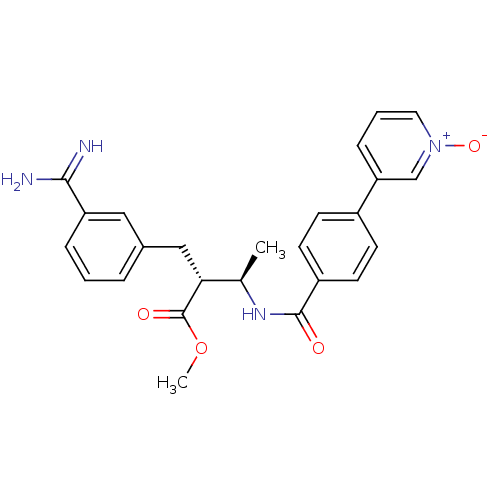
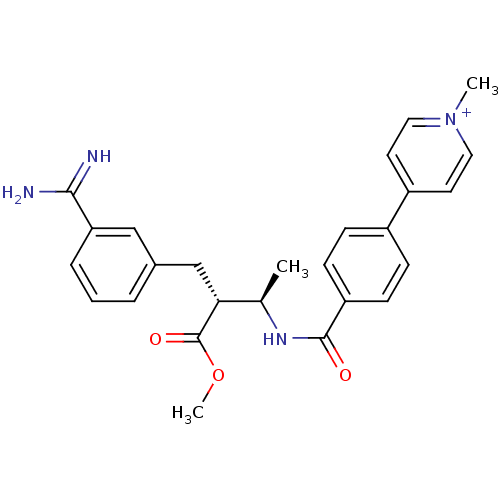
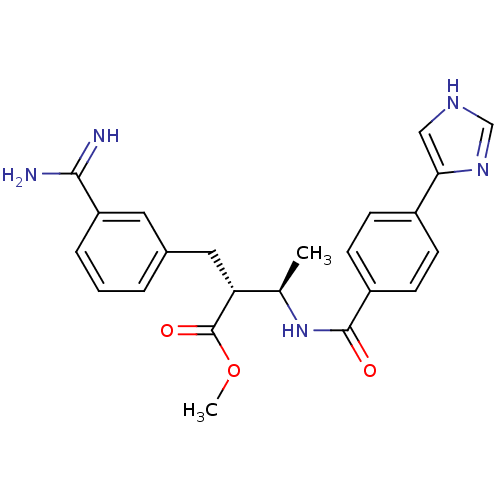
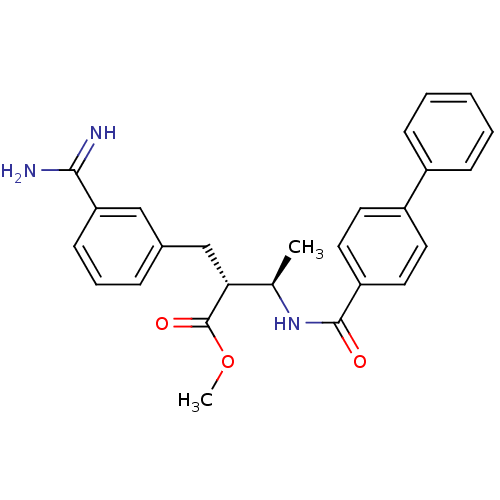
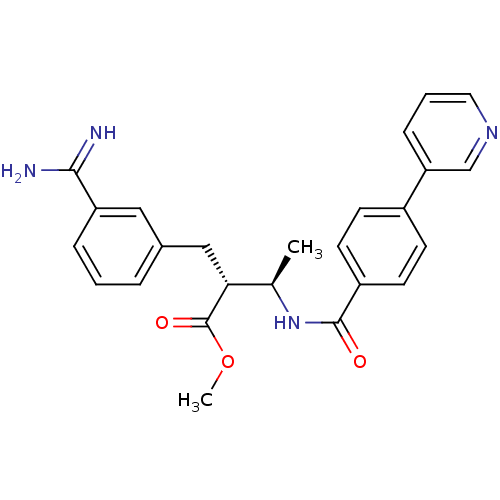
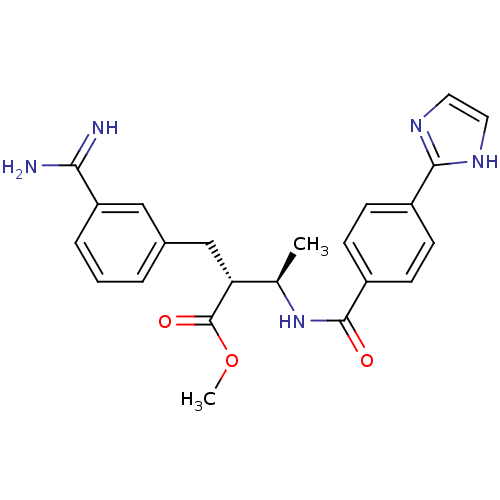
Affinity DataKi: 0.400nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.580nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inibition of Coagulation factor XaMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 69nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 76nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 168nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 301nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 368nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >2.90E+3nMAssay Description:In vitro inhibitory potency against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.25E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of Coagulation factor IIaMore data for this Ligand-Target Pair