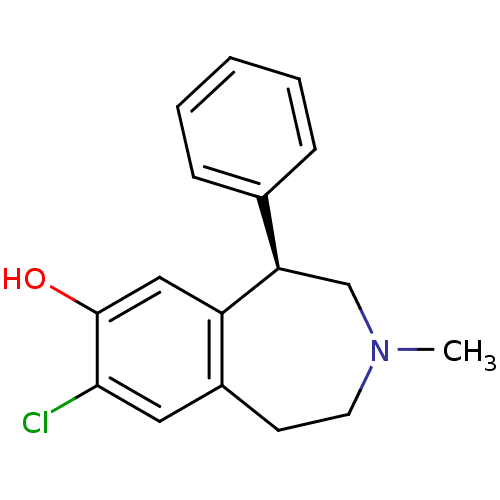
Affinity DataKi: 0.300nMAssay Description:Compound was tested for the displacement of [3H]-SCH- 23390 from dopamine receptor D1More data for this Ligand-Target Pair
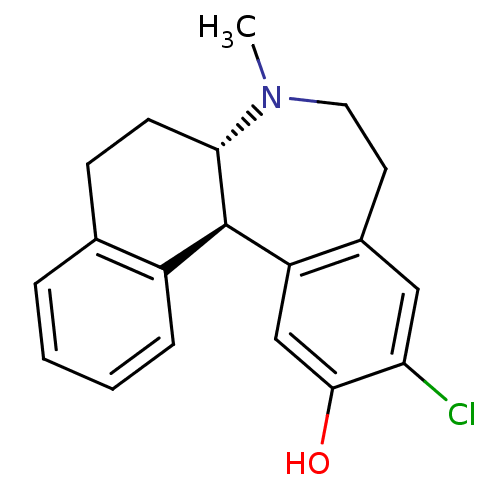
Affinity DataKi: 1.90nMAssay Description:Compound was tested for the displacement of [3H]-SCH- 23390 from dopamine receptor D1More data for this Ligand-Target Pair
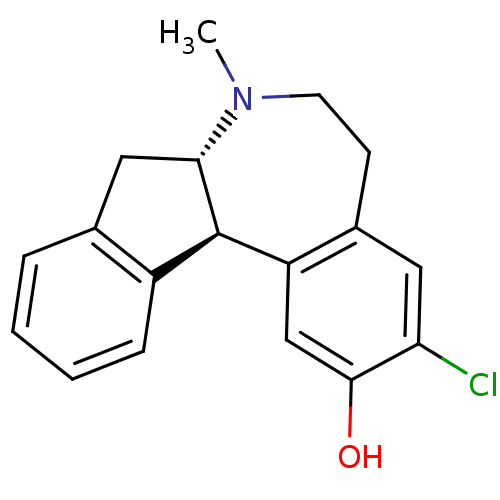
Affinity DataKi: 7nMAssay Description:Compound was tested for the displacement of [3H]-SCH- 23390 from dopamine receptor D1More data for this Ligand-Target Pair
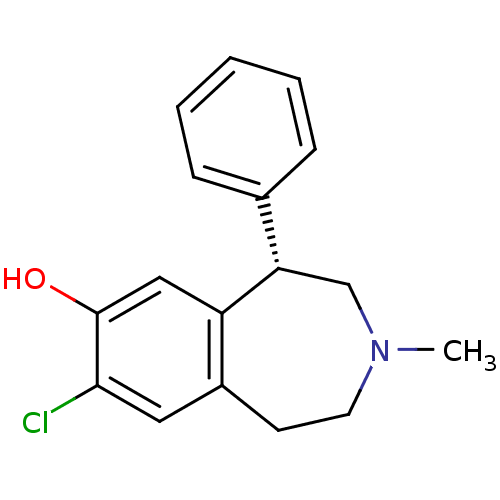
Affinity DataKi: 192nMAssay Description:Compound was tested for the displacement of [3H]-SCH- 23390 from dopamine receptor D1More data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
University Of Lund
Curated by ChEMBL
University Of Lund
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Compound was tested for the adenylate cyclase stimulationMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
University Of Lund
Curated by ChEMBL
University Of Lund
Curated by ChEMBL
Affinity DataEC50: 71nMAssay Description:Compound was tested for the adenylate cyclase stimulationMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
University Of Lund
Curated by ChEMBL
University Of Lund
Curated by ChEMBL
Affinity DataEC50: 5.20E+3nMAssay Description:Compound was tested for the adenylate cyclase stimulationMore data for this Ligand-Target Pair