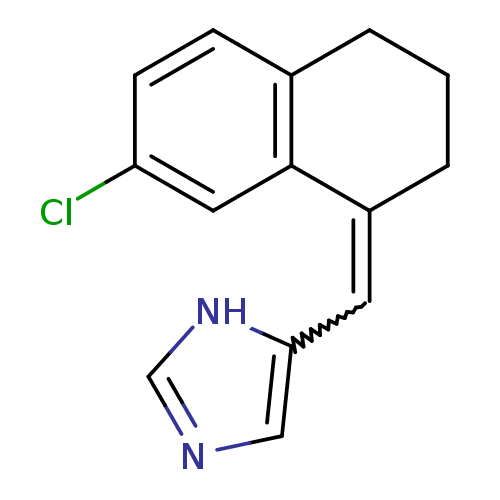
Affinity DataIC50: 1nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 9.60nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 9.70nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 10.3nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 11.2nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 12.3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 13.9nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 16.7nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 18.7nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 19.5nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 20.6nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 22.7nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 23.5nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 24.8nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 25.9nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 26.2nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 28.7nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 31.4nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 35.7nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 35.9nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 47.3nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 88.8nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 92.8nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 119nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 218nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 226nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 810nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 955nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataIC50: 970nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)