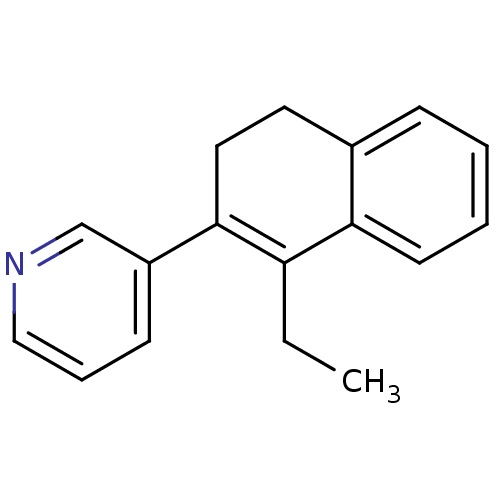
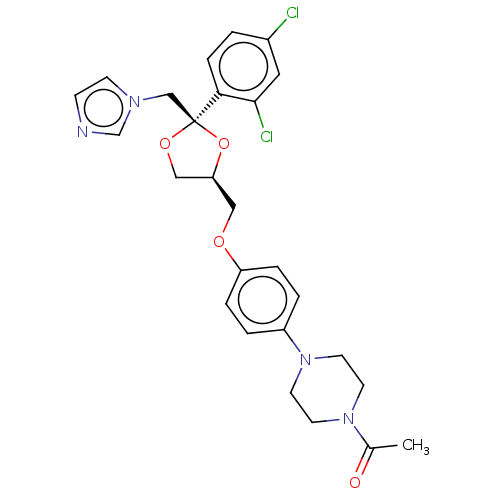
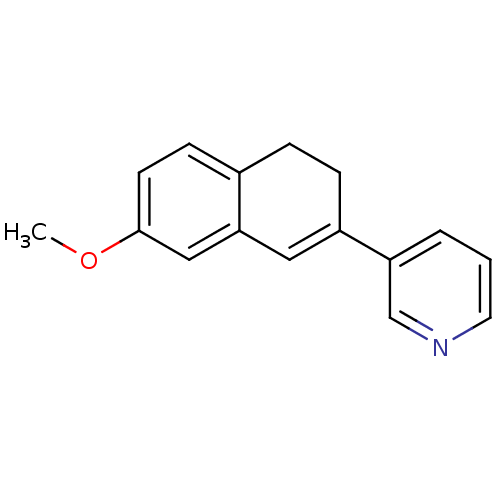
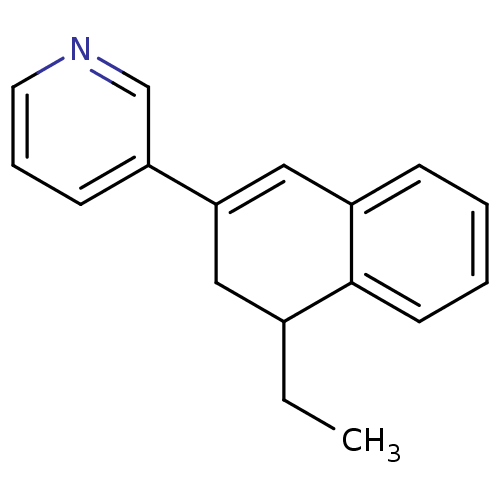
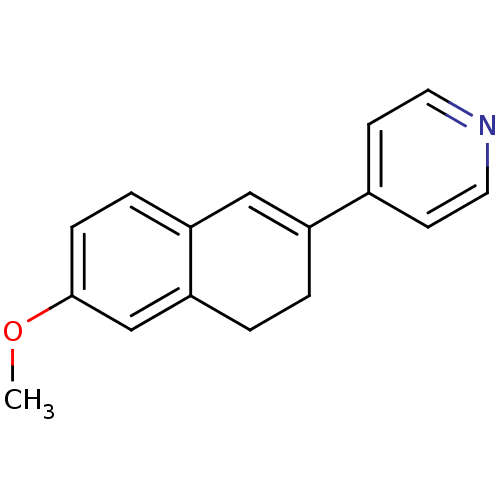
Affinity DataKi: 1.30nM IC50: 2nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nM IC50: 4nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nM IC50: 7nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataKi: 8.40nM IC50: 13nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 224nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 288nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 334nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 411nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 448nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 503nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 578nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 639nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 735nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 763nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 814nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 1.09E+3nMAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 1.62E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.73E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.39E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.53E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.83E+3nMAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 3.55E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 4.07E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 5.68E+3nMpH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro...More data for this Ligand-Target Pair
Affinity DataIC50: 6.05E+3nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: >3.60E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: >3.60E+4nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from 3H-labeled androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair
Affinity DataAssay Description:The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr...More data for this Ligand-Target Pair

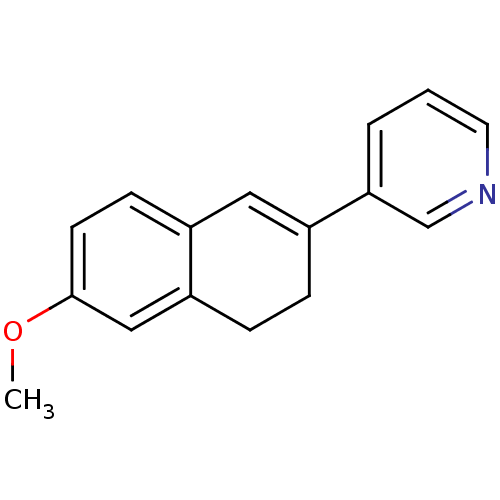

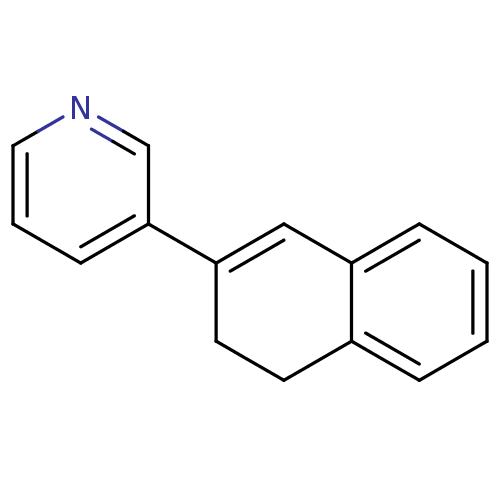
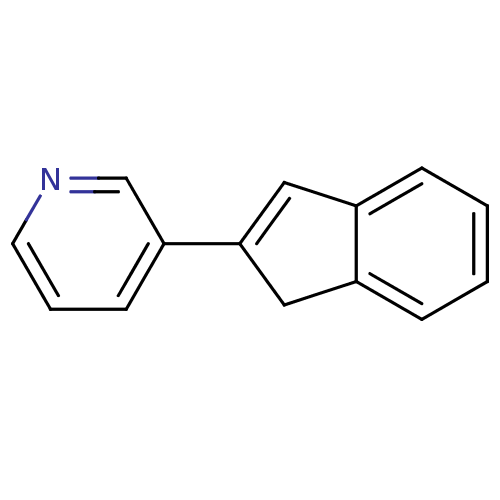
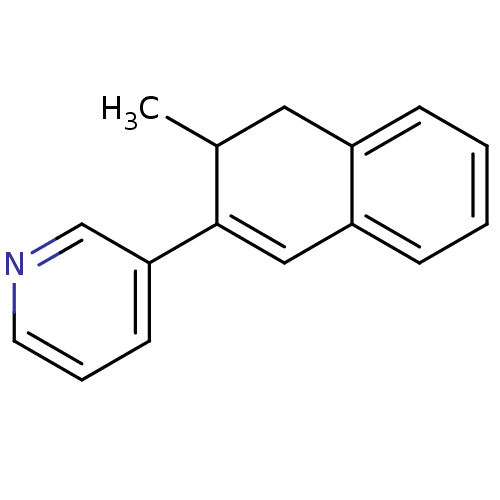

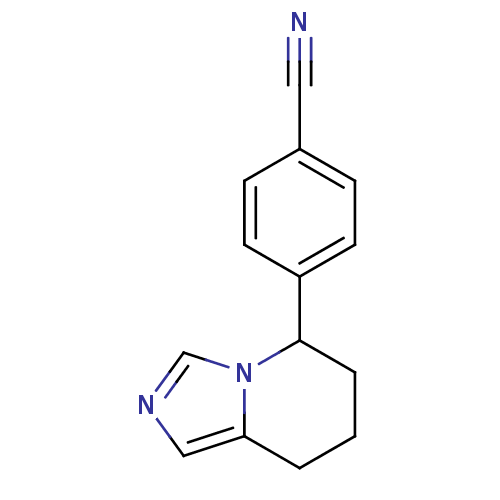
 3D Structure (crystal)
3D Structure (crystal)