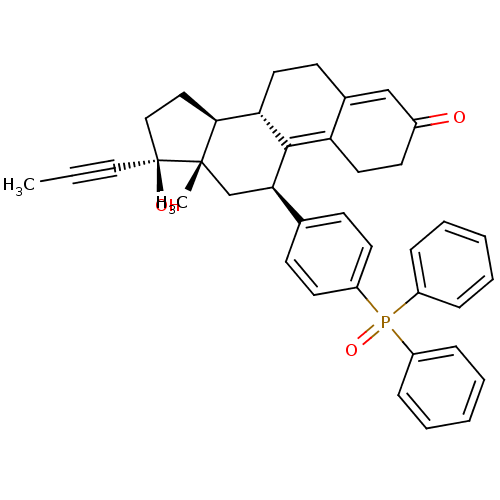
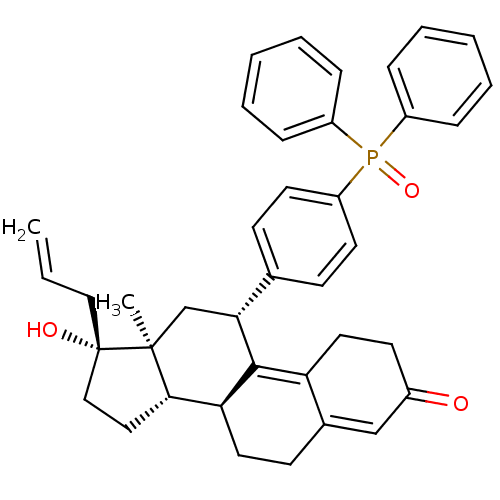
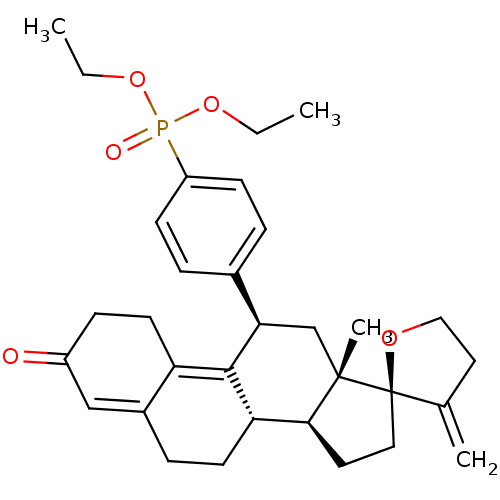
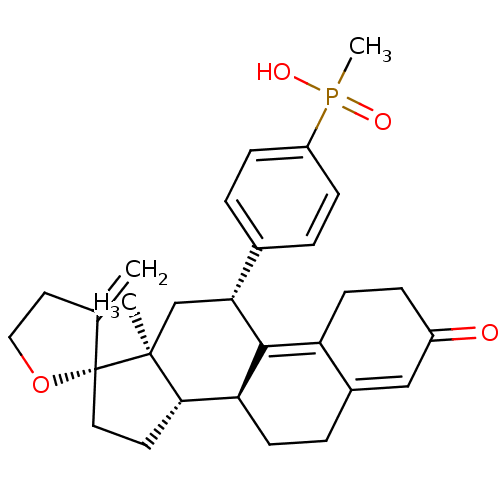
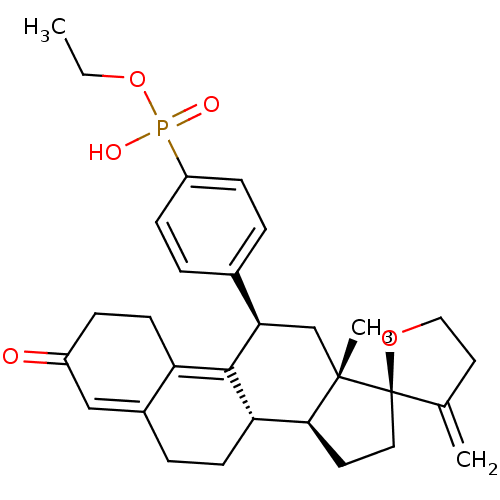
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 56.3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 59.8nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >3.00E+3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >3.00E+3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 9.90nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 101nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 141nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 275nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 308nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 381nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 763nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)