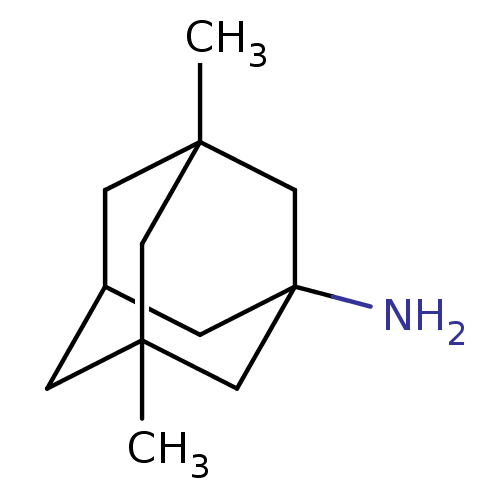
Affinity DataIC50: 1.54nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.65nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.15nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.57nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 21.5nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 45.8nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 189nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 211nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 296nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 424nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons...More data for this Ligand-Target Pair
Affinity DataIC50: 2.58E+3nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataIC50: 9.52E+3nMAssay Description:Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataIC50: 1.82E+4nMAssay Description:Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1(Homo sapiens (Human))
University Of Bologna
Curated by ChEMBL
University Of Bologna
Curated by ChEMBL
Affinity DataIC50: 3.03E+4nMAssay Description:Inhibition of NMDA NR1/NR2B receptor (unknown origin) expressed in xenopus oocytes assessed as inhibition of NMDA and glycine-induced current respons...More data for this Ligand-Target Pair






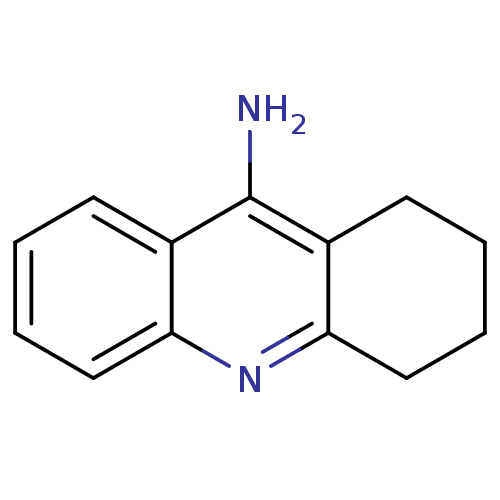
 3D Structure (crystal)
3D Structure (crystal)