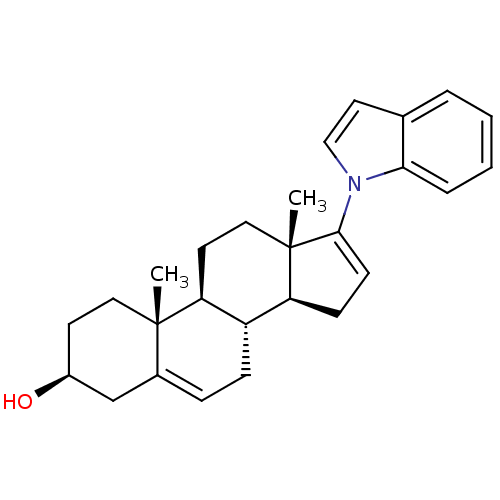
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 125nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
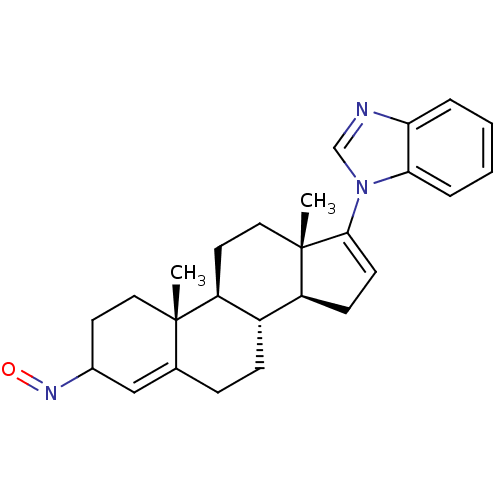
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 206nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
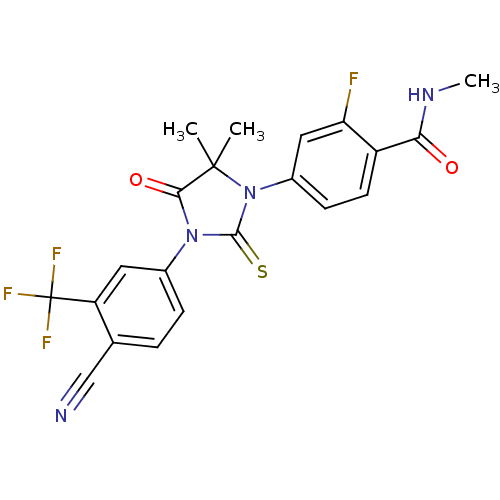
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 752nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
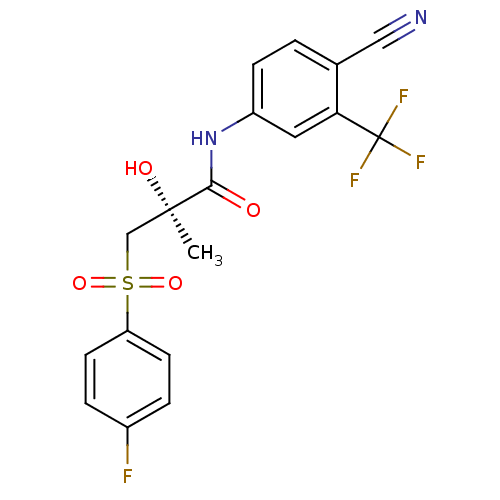
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 9.37E+4nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 1.22E+5nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 1.32E+5nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataIC50: 2.58E+5nMAssay Description:Inhibition of human truncated CYP17A1 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataEC50: 49nMAssay Description:Displacement of [3H]R1881 from AR in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 31nMAssay Description:Displacement of [3H]R1881 from AR in human MDA-MB-453 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 670nMAssay Description:Displacement of [3H]R1881 from AR in human LNCaP cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 915nMAssay Description:Displacement of [3H]R1881 from AR in human LNCaP cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+3nMAssay Description:Displacement of [3H]R1881 from AR in human LNCaP cells after 2 hrs by scintillation counting analysisMore data for this Ligand-Target Pair

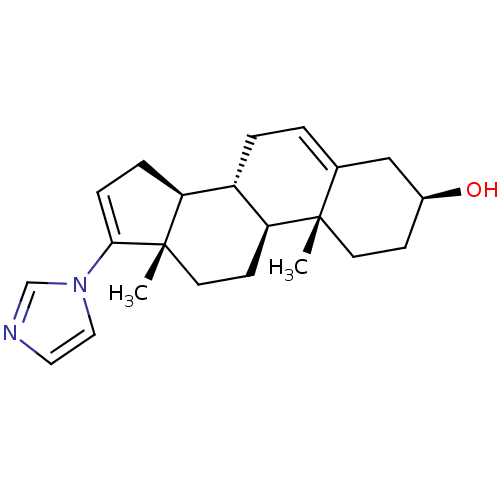
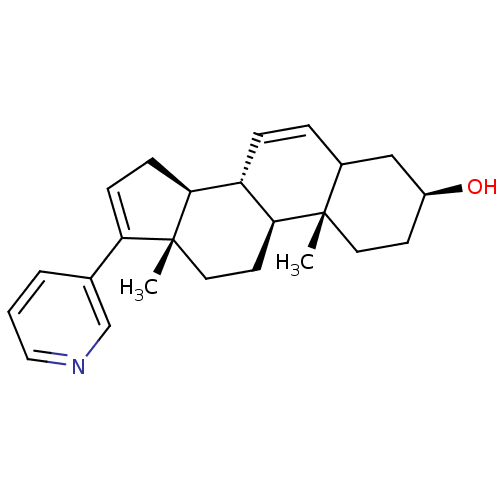
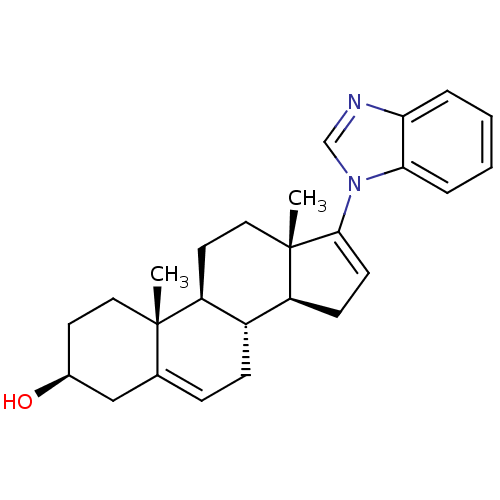
 3D Structure (crystal)
3D Structure (crystal)