TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.000650nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0180nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8.10nMAssay Description:Binding affinity of the compound against human alpha trypsinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8.10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9.60nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity of the compound against human alpha trypsinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 85nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.23E+4nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Binding activity of the compound against human alpha thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Compound was tested for inhibition of gel-filtered platelet (GFP) aggregation induced by alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Compound was tested for inhibition of gel-filtered platelet (GFP) aggregation induced by alpha-thrombinMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 530nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibitory activity of the compound against human Cathepsin GMore data for this Ligand-Target Pair

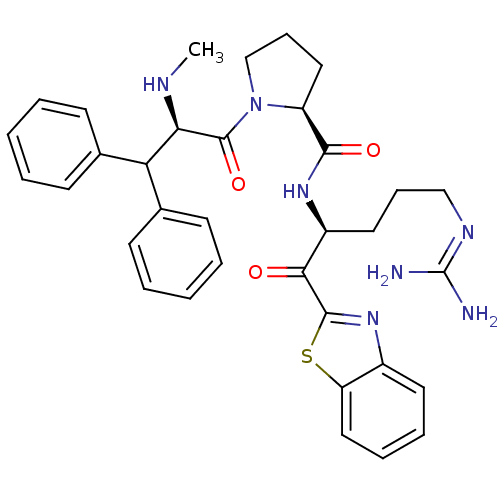
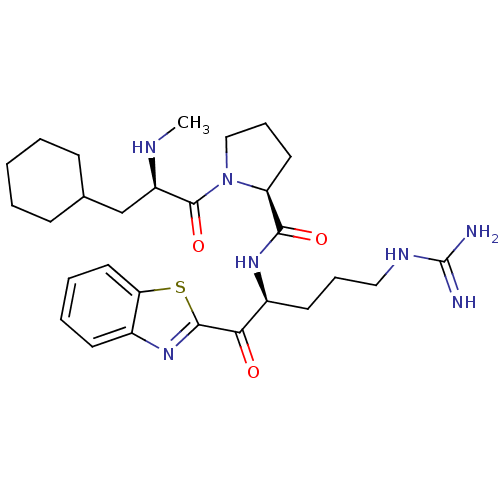
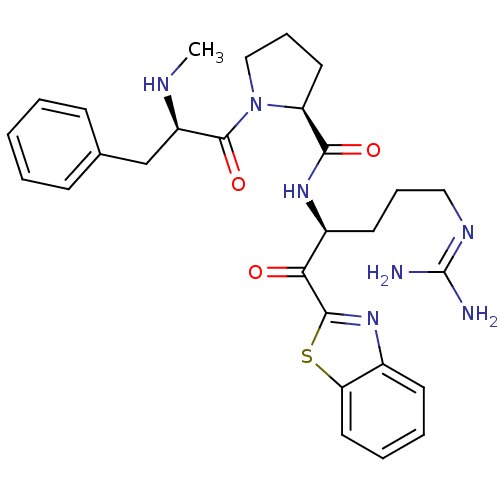
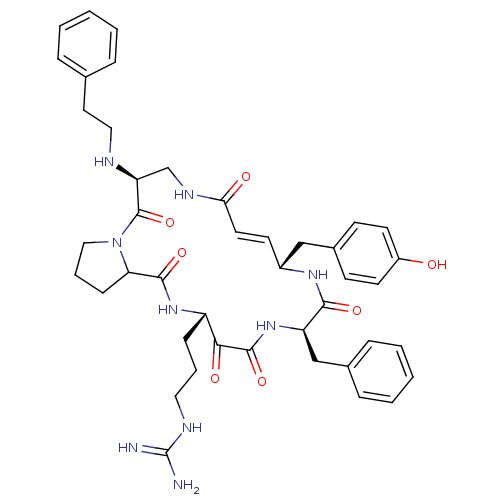

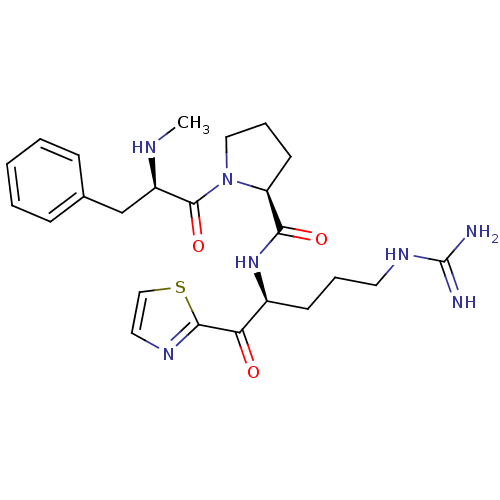
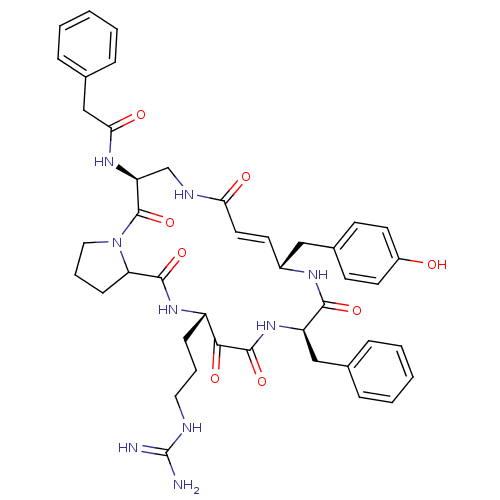
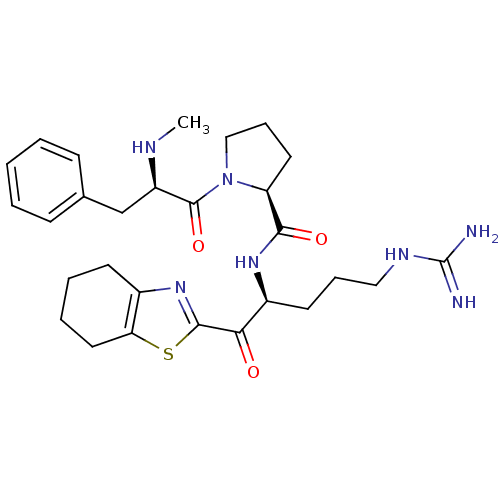
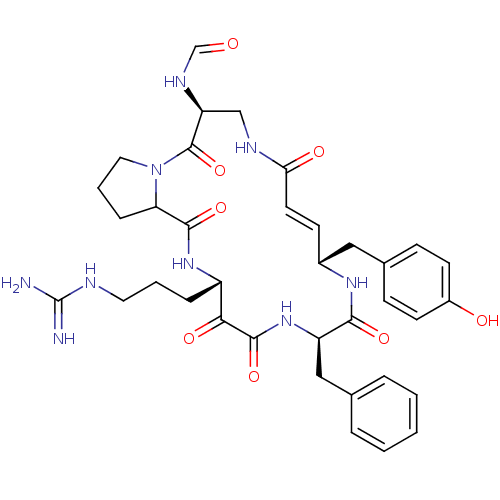






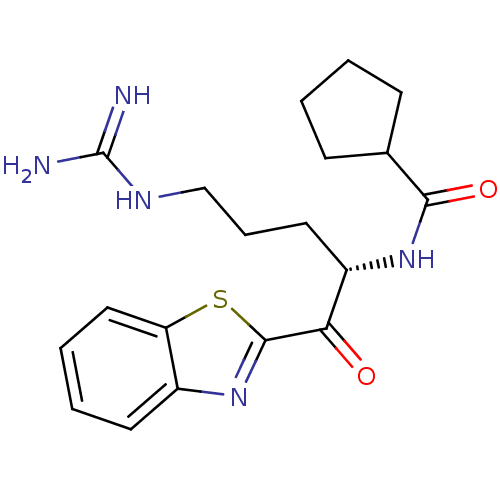




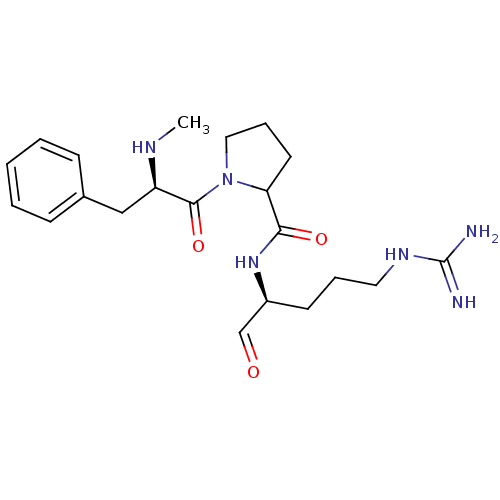

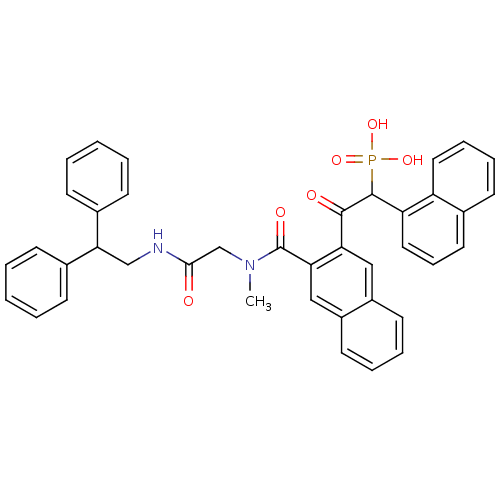


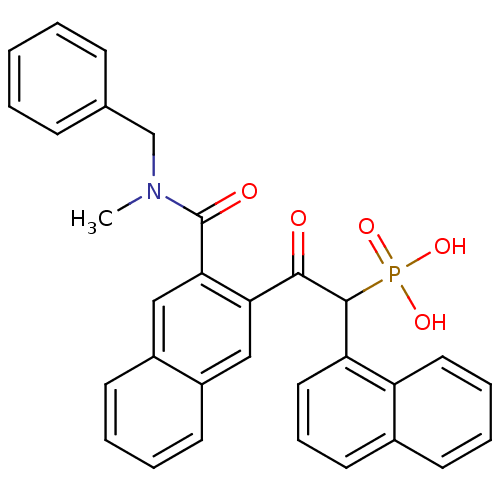


 3D Structure (crystal)
3D Structure (crystal)