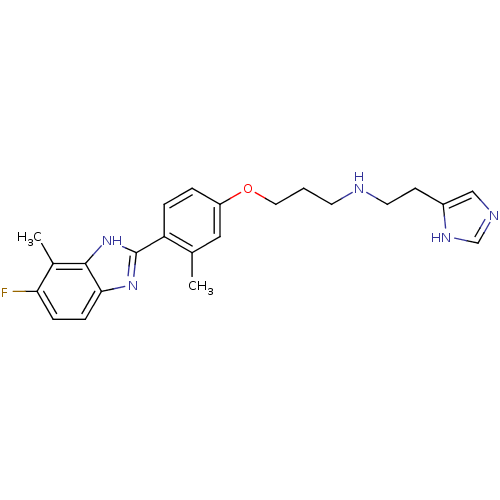
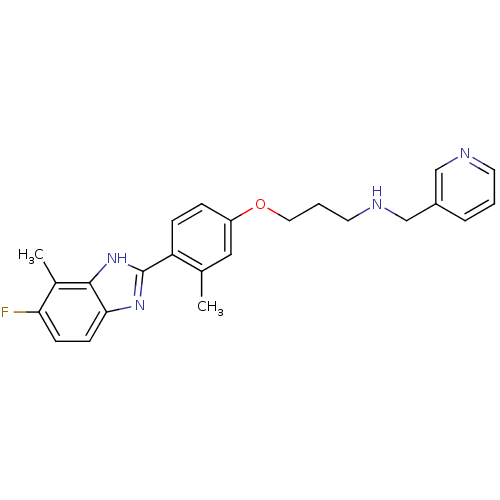
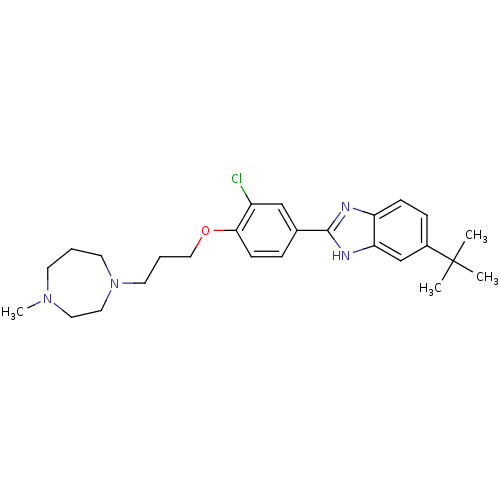
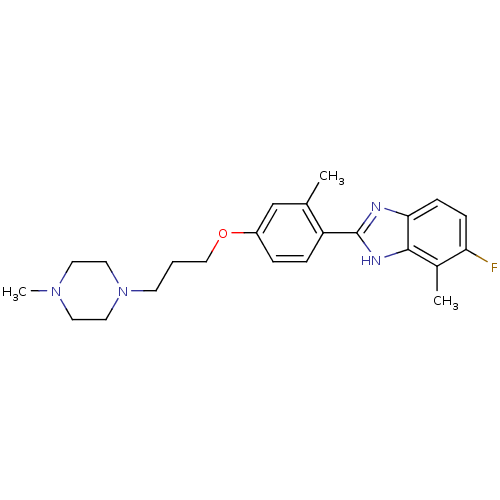
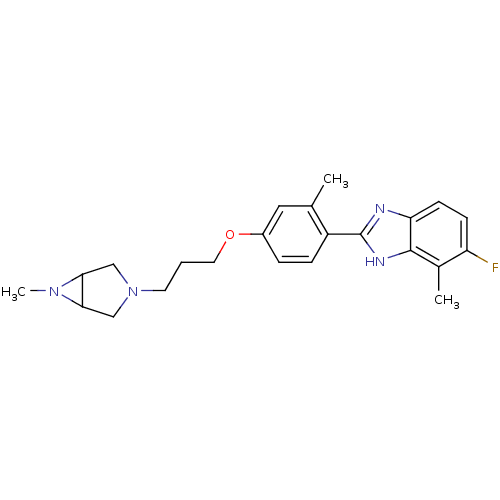
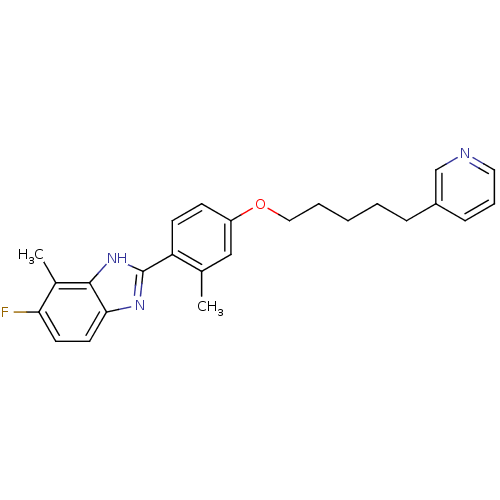
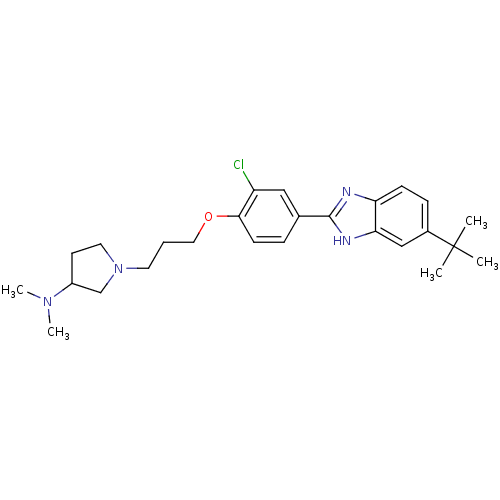
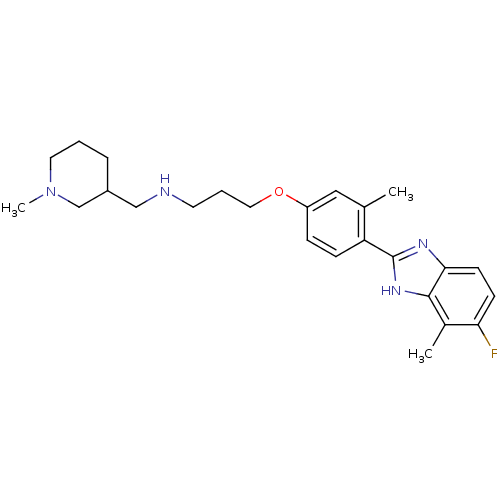
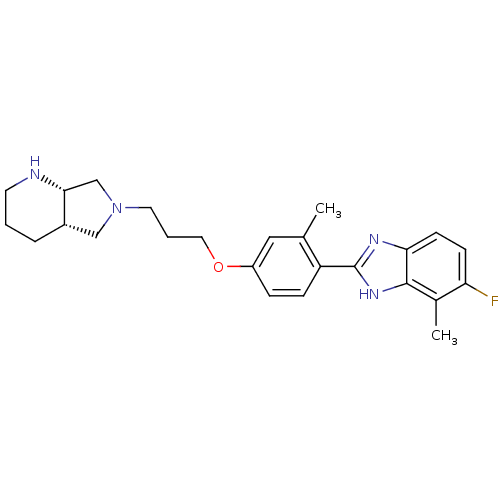
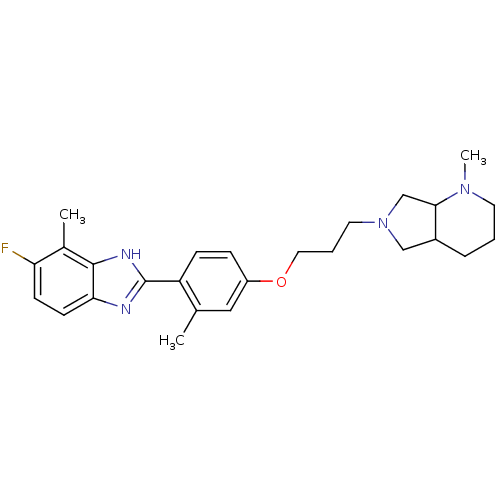
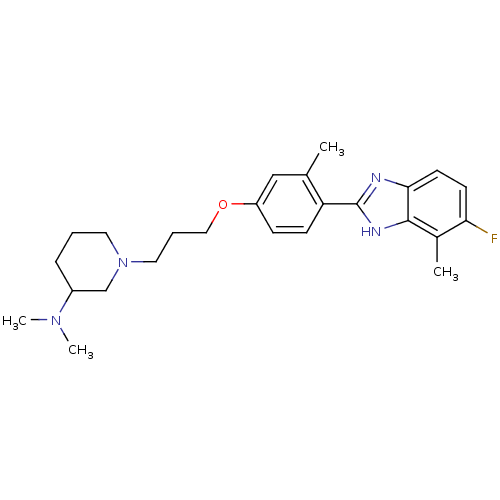
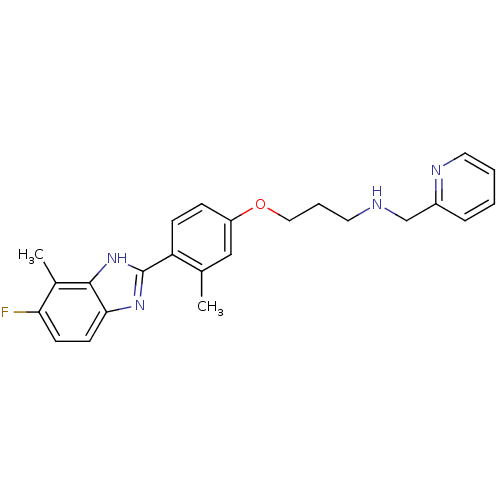
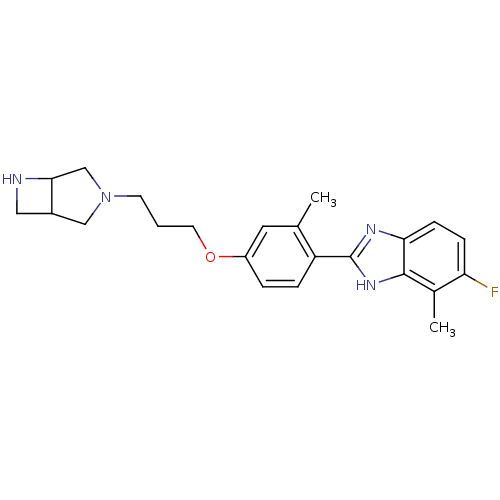
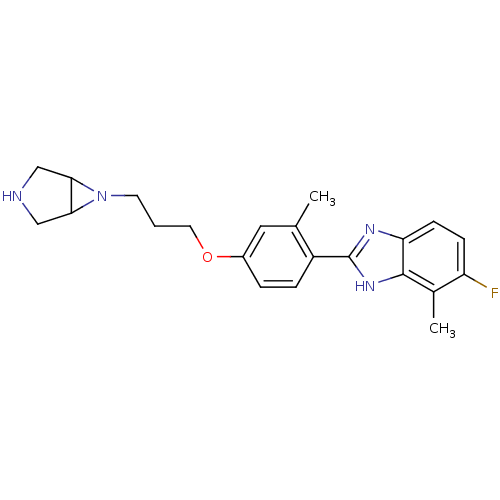
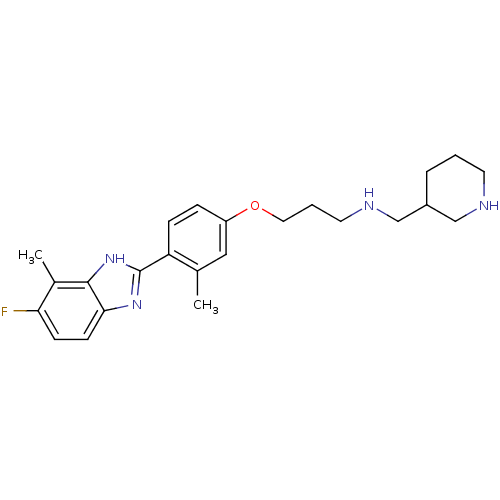
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.210nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5.30nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 42nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 72nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 77nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 84nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 133nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 152nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 228nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 232nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 250nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 557nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 609nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 691nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 971nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.76E+3nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.30nMAssay Description:Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 0.560nMAssay Description:Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Mus musculus (mouse))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 39nMAssay Description:Intrinsic activity at mouse histamine H4 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP responsive elemen...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 6.90nMAssay Description:Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.30nMAssay Description:Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Mus musculus (mouse))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 12nMAssay Description:Displacement of [3H]histamine from mouse recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Mus musculus (mouse))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 548nMAssay Description:Displacement of [3H]histamine from mouse recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Mus musculus (mouse))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.20E+3nMAssay Description:Displacement of [3H]histamine from mouse recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 377nMAssay Description:Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 9.00E+3nMAssay Description:Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 119nMAssay Description:Displacement of [125H]iodoproxyphan from human recombinant histamine H3 receptor expressed in human SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 131nMAssay Description:Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Displacement of [125I]APT from human recombinant histamine H2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor expressed in human SK-N-MC cellsMore data for this Ligand-Target Pair