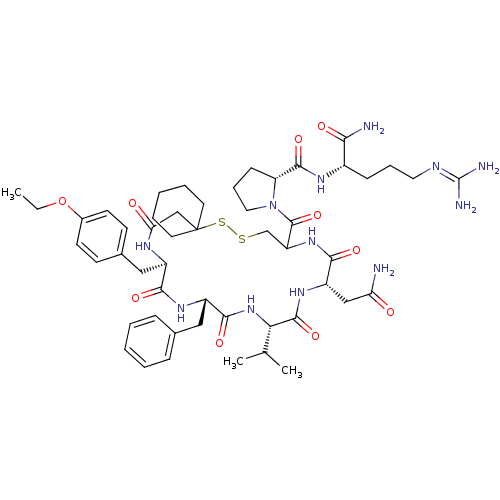
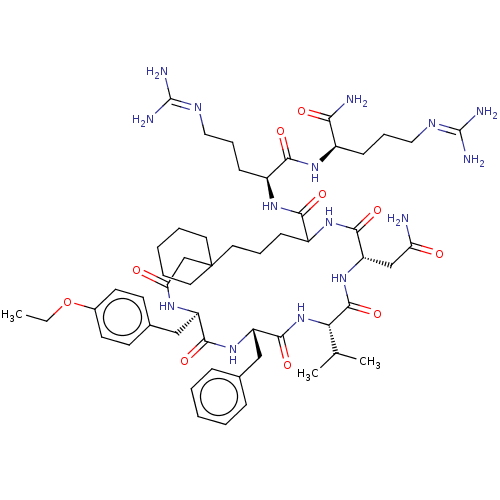
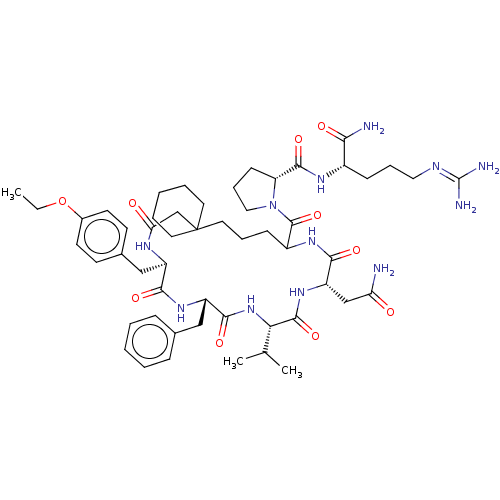
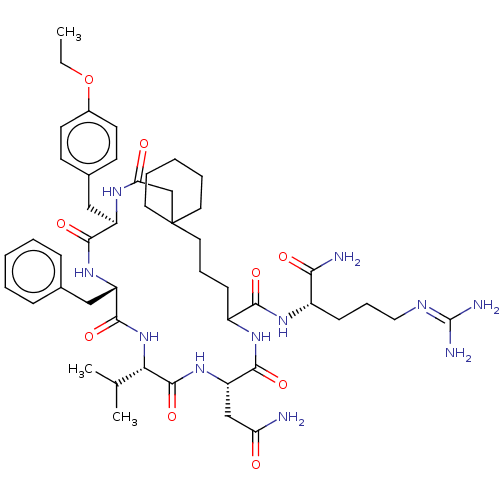
Affinity DataKi: 3.60nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 4.00E+5nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+5nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 5.00E+5nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 1.10E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 1.50E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 2.40E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 2.70E+6nMAssay Description:In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in humanMore data for this Ligand-Target Pair
Affinity DataKi: 2.70E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 3.60E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 3.90E+6nMAssay Description:In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in humanMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/2/3/4/5/6/7/8/9(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 4.40E+6nMAssay Description:In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in...More data for this Ligand-Target Pair