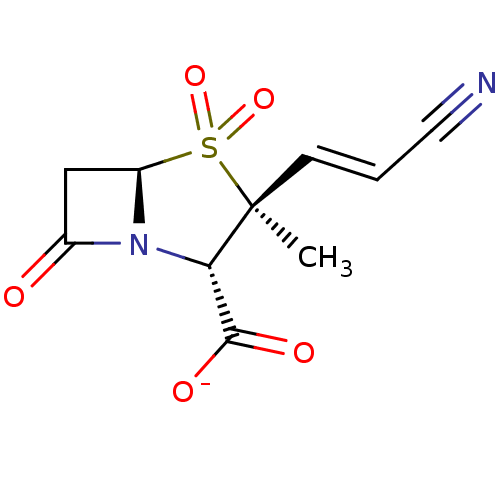
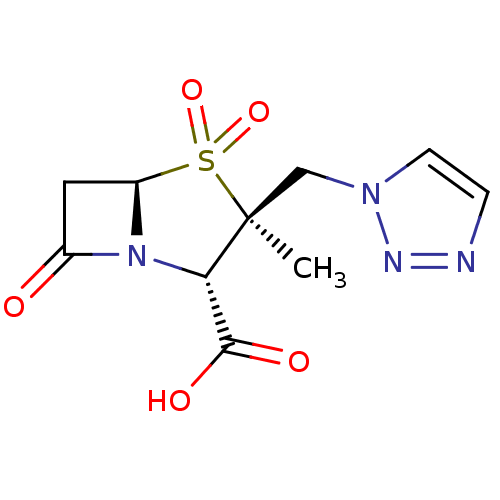
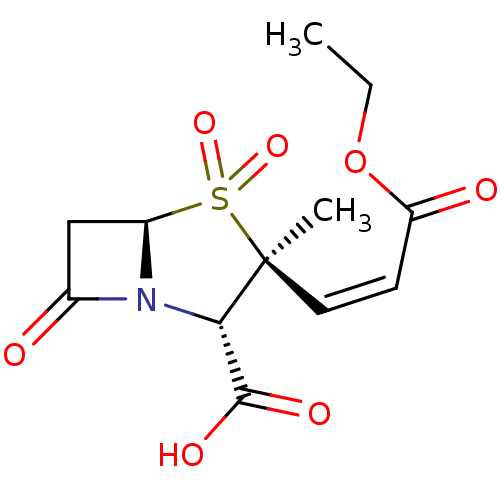
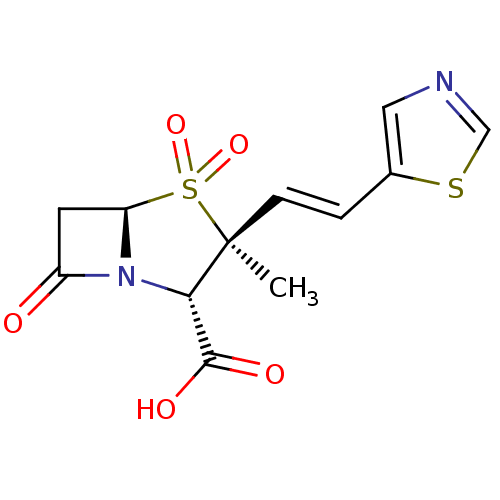
Affinity DataIC50: 90nMAssay Description:Compound was tested for inhibition of Beta-lactamase from PSE-1More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was tested for inhibition of Beta-lactamase from OXA-1More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was tested for inhibition of Beta-lactamase from OXA-1More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-3More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-3More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Compound was tested for inhibition of beta-Lactamases from Morganell morgani U1627More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Compound was tested for inhibition of Beta-lactamase fromOXA-2More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Morganell morgani U1627More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Citrobacter freundii 1982More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Compound was tested for inhibition of Beta-lactamase from OXA-1More data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Staphylococcus aureus PCIMore data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Bacillus licheniformis 749/CMore data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SHMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SHMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Enterobacter cloacae 908RMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-1More data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 490nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Staphylococcus aureus PCIMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Bacillus licheniformis 749/CMore data for this Ligand-Target Pair
TargetBeta-lactamase SHV-1(Klebsiella pneumoniae (Enterobacteria))
F. Hoffmann-La Roche
Curated by ChEMBL
F. Hoffmann-La Roche
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Compound was tested for inhibition of Beta-lactamase from SHV-2More data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-2More data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Compound was tested for inhibition of Beta-lactamase from PSE-1More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa 18SHMore data for this Ligand-Target Pair
Affinity DataIC50: 830nMAssay Description:Compound was tested for inhibition of Beta-lactamase fromOXA-2More data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Compound was tested for inhibition of Beta-lactamase from SHV-2More data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Citrobacter freundii 1982More data for this Ligand-Target Pair
Affinity DataIC50: 980nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.07E+3nMAssay Description:Compound was tested for inhibition of Beta-lactamase from RTEM-1More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Compound was tested for inhibition of Escherichia coli AmpC ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Compound was tested for inhibition of Escherichia coli AmpC ,class C of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Enterobacter cloacae 908RMore data for this Ligand-Target Pair
Affinity DataIC50: 1.97E+3nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa MK-1184More data for this Ligand-Target Pair
Affinity DataIC50: 2.11E+3nMAssay Description:Compound was tested for inhibition of Beta-lactamase from Pseudomonas aeruginosa GN10362More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamaseMore data for this Ligand-Target Pair