Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataCell Reactant:
Transcriptional Regulator TtgR
Syringe Reactant:
BDBM7459
Meas. Tech.:
Isothermal Titration Calorimetry
Entry Date.:
07/23/08
ΔG°:
-7.13±0.03 (kcal/mole)
pH:
7±n/a
Log10Kb:
3.8
Temperature:
303.15±n/a (K)
ΔHobs :
-3.05±0.09 (kJ/mole)
Corrected for ΔHioniz:
no
ΔS° :
0.01±0 (kJ/mole-K)
Citation
Cell React
Source:
A 651-bp fragment containing the ttgR gene was amplified by PCR from P. putida DOT-T1E chromosomal DNA, and cloned in E. coli expression vector. TtgR protein was over-expressed and purified.
Prep. Method:
TtgR protein was further purified by size exclusion chromatography using a Sephacryl HR-200 column (Amersham Biosciences). Eluted fractions of TtgR were pooled, concentrated, and dialyzed against the buffer.
Name:
Transcriptional Regulator TtgR
Synonyms:
HTH-type transcriptional regulator ttgR | TTGR_PSEPT | Toluene efflux pump ttgABC operon repressor | ttgR
Type:
Repressor; homodimer
Mol. Mass.:
23852.57
Organism:
Pseudomonas putida
Description:
n/a
Residue:
210
Sequence:
MVRRTKEEAQETRAQIIEAAERAFYKRGVARTTLADIAELAGVTRGAIYWHFNNKAELVQALLDSLHETHDHLARASESEDEVDPLGCMRKLLLQVFNELVLDARTRRINEILHHKCEFTDDMCEIRQQRQSAVLDCHKGITLALANAVRRGQLPGELDAERAAVAMFAYVDGLIRRWLLLPDSVDLLGDVEKWVDTGLDMLRLSPALRK
Syringe React
Prep. Method:
All chemicals were manipulated in glass vessels and effector samples were neither degassed nor filtered, to avoid evaporation or nonspecific binding. Analyses were carried out using 5% DMSO to facilitate solubilization.
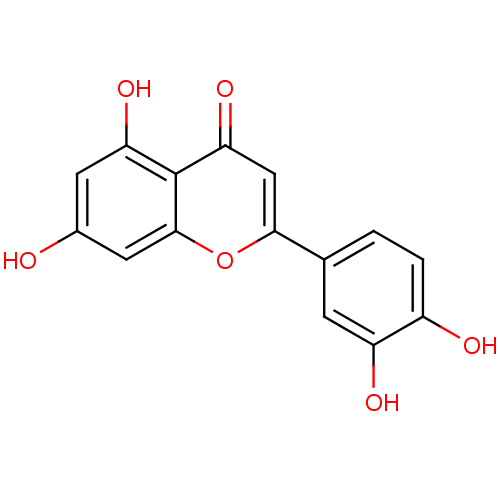
Name:
BDBM7459
Synonyms:
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-chromen-4-one | CHEMBL151 | Luteolin (27) | Luteolin (4) | acs.jmedchem.1c00409_ST.600 | cid_5280445 | luteolin | med.21724, Compound 3
Type:
Small organic molecule
Emp. Form.:
C15H10O6
Mol. Mass.:
286.2363
SMILES:
Oc1cc(O)c2c(c1)oc(cc2=O)-c1ccc(O)c(O)c1