Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Dipeptidyl peptidase 4
Ligand
BDBM11543
Substrate
BDBM11526
Meas. Tech.
In Vitro DPP-IV Inhibition Assays
pH
7.4±n/a
Temperature
295.15±n/a K
Ki
2.1±0.3 nM
Comments
The compound did not show any significant inhibition of DPP II at concentration up to 30 uM.
Citation
 Augeri, DJ; Robl, JA; Betebenner, DA; Magnin, DR; Khanna, A; Robertson, JG; Wang, A; Simpkins, LM; Taunk, P; Huang, Q; Han, SP; Abboa-Offei, B; Cap, M; Xin, L; Tao, L; Tozzo, E; Welzel, GE; Egan, DM; Marcinkeviciene, J; Chang, SY; Biller, SA; Kirby, MS; Parker, RA; Hamann, LG Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48:5025-37 (2005) [PubMed] Article
Augeri, DJ; Robl, JA; Betebenner, DA; Magnin, DR; Khanna, A; Robertson, JG; Wang, A; Simpkins, LM; Taunk, P; Huang, Q; Han, SP; Abboa-Offei, B; Cap, M; Xin, L; Tao, L; Tozzo, E; Welzel, GE; Egan, DM; Marcinkeviciene, J; Chang, SY; Biller, SA; Kirby, MS; Parker, RA; Hamann, LG Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48:5025-37 (2005) [PubMed] Article Target
Name:
Dipeptidyl peptidase 4
Synonyms:
ADABP | ADCP2 | Adenosine deaminase complexing protein 2 | CD26 | CD_antigen=CD26 | DPP IV | DPP4 | DPP4_HUMAN | DPPIV | Dipeptidyl peptidase 4 (DDP-IV) | Dipeptidyl peptidase 4 (DPP IV) | Dipeptidyl peptidase 4 (DPP-4) | Dipeptidyl peptidase 4 (DPP4) | Dipeptidyl peptidase 4 (DPPIV) | Dipeptidyl peptidase 4 membrane form | Dipeptidyl peptidase 4 soluble form | Dipeptidyl peptidase IV | Dipeptidyl peptidase IV (DDP-4) | Dipeptidyl peptidase IV (DDP-IV) | Dipeptidyl peptidase IV (DPP IV) | Dipeptidyl peptidase IV membrane form | Dipeptidyl peptidase IV soluble form | Dipeptidyl peptidase-IV (DPP-4) | Dipeptidyl peptidase-IV (DPP-IV) | T-cell activation antigen CD26 | TP103
Type:
Enzyme
Mol. Mass.:
88271.01
Organism:
Homo sapiens (Human)
Description:
P27487
Residue:
766
Sequence:
MKTPWKVLLGLLGAAALVTIITVPVVLLNKGTDDATADSRKTYTLTDYLKNTYRLKLYSLRWISDHEYLYKQENNILVFNAEYGNSSVFLENSTFDEFGHSINDYSISPDGQFILLEYNYVKQWRHSYTASYDIYDLNKRQLITEERIPNNTQWVTWSPVGHKLAYVWNNDIYVKIEPNLPSYRITWTGKEDIIYNGITDWVYEEEVFSAYSALWWSPNGTFLAYAQFNDTEVPLIEYSFYSDESLQYPKTVRVPYPKAGAVNPTVKFFVVNTDSLSSVTNATSIQITAPASMLIGDHYLCDVTWATQERISLQWLRRIQNYSVMDICDYDESSGRWNCLVARQHIEMSTTGWVGRFRPSEPHFTLDGNSFYKIISNEEGYRHICYFQIDKKDCTFITKGTWEVIGIEALTSDYLYYISNEYKGMPGGRNLYKIQLSDYTKVTCLSCELNPERCQYYSVSFSKEAKYYQLRCSGPGLPLYTLHSSVNDKGLRVLEDNSALDKMLQNVQMPSKKLDFIILNETKFWYQMILPPHFDKSKKYPLLLDVYAGPCSQKADTVFRLNWATYLASTENIIVASFDGRGSGYQGDKIMHAINRRLGTFEVEDQIEAARQFSKMGFVDNKRIAIWGWSYGGYVTSMVLGSGSGVFKCGIAVAPVSRWEYYDSVYTERYMGLPTPEDNLDHYRNSTVMSRAENFKQVEYLLIHGTADDNVHFQQSAQISKALVDVGVDFQAMWYTDEDHGIASSTAHQHIYTHMSHFIKQCFSLP
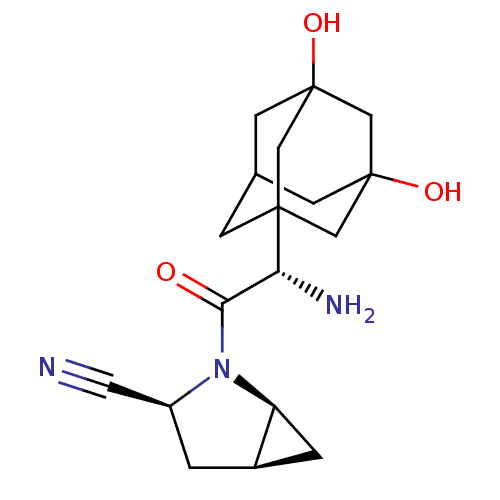
Inhibitor
Name:
BDBM11543
Synonyms:
(1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid | (S)-3,5-Dihydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt | BMS-477118 | Saxagliptin analogue 28
Type:
Small organic molecule
Emp. Form.:
C18H25N3O3
Mol. Mass.:
331.4094
SMILES:
N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(O)(CC(O)(C3)C1)C2 |r,TLB:21:14:23:18.22.19,21:19:14.15.13:23,20:19:14.15.13:23,17:16:14.13.21:22,15:16:14.13.21:22,THB:15:14:22:18.23.16|