Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
Ligand
BDBM13954
Substrate
BDBM13466
Meas. Tech.
PTP1B and TCPTP Inhibition Assay
pH
7.5±n/a
Temperature
295.15±n/a K
Ki
22±6 nM
Citation
 Szczepankiewicz, BG; Liu, G; Hajduk, PJ; Abad-Zapatero, C; Pei, Z; Xin, Z; Lubben, TH; Trevillyan, JM; Stashko, MA; Ballaron, SJ; Liang, H; Huang, F; Hutchins, CW; Fesik, SW; Jirousek, MR Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J Am Chem Soc 125:4087-96 (2003) [PubMed] Article
Szczepankiewicz, BG; Liu, G; Hajduk, PJ; Abad-Zapatero, C; Pei, Z; Xin, Z; Lubben, TH; Trevillyan, JM; Stashko, MA; Ballaron, SJ; Liang, H; Huang, F; Hutchins, CW; Fesik, SW; Jirousek, MR Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J Am Chem Soc 125:4087-96 (2003) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
Synonyms:
PTN1_HUMAN | PTP-1B | PTP1B | PTPN1 | PTPase 1B | Protein-Tyrosine Phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase, non-receptor type 1
Type:
Enzyme
Mol. Mass.:
34670.65
Organism:
Homo sapiens (Human)
Description:
The catalytic domain of PTP 1B (residues 1-298) was expressed and purified from E. coli.
Residue:
298
Sequence:
MEMEKEFEQIDKSGSWAAIYQDIRHEASDFPCRVAKLPKNKNRNRYRDVSPFDHSRIKLHQEDNDYINASLIKMEEAQRSYILTQGPLPNTCGHFWEMVWEQKSRGVVMLNRVMEKGSLKCAQYWPQKEEKEMIFEDTNLKLTLISEDIKSYYTVRQLELENLTTQETREILHFHYTTWPDFGVPESPASFLNFLFKVRESGSLSPEHGPVVVHCSAGIGRSGTFCLADTCLLLMDKRKDPSSVDIKKVLLEMRKFRMGLIQTADQLRFSYLAVIEGAKFIMGDSSVQDQWKELSHED
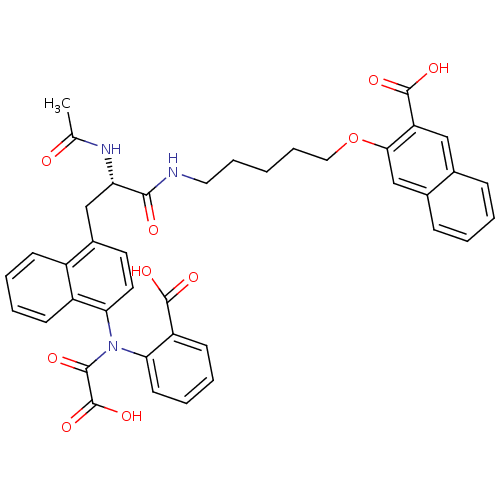
Inhibitor
Name:
BDBM13954
Synonyms:
3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic acid]naphthalen-1-yl}-2-acetamidopropanamido]pentyl}oxy)naphthalene-2-carboxylic acid | 3-({5-[(N-Acetyl-3-{4-[(carboxycarbonyl)(2-carboxyphenyl)amino]-1-naphthyl}-L-alanyl)amino]pentyl}oxy)-2-naphthoic Acid | linked-fragment derived compound 23
Type:
Small organic molecule
Emp. Form.:
C40H37N3O10
Mol. Mass.:
719.7359
SMILES:
CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r|
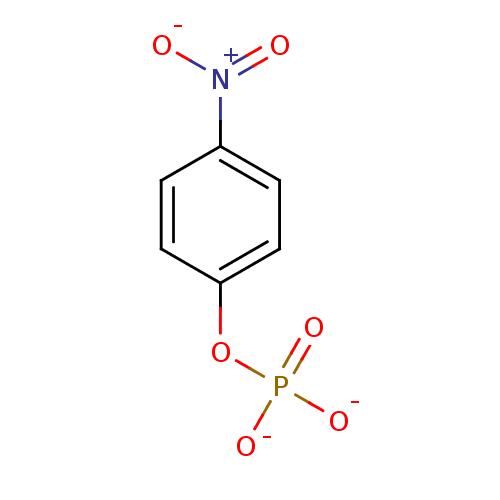
Substrate
Name:
BDBM13466
Synonyms:
4-Nitrophenyl phosphate disodium salt hexahydrate | 4-nitrophenyl phosphate (pNPP) | disodium (4-nitrophenyl) phosphate | para-nitrophenyl phosphate (pNPP)
Type:
Small organic molecule
Emp. Form.:
C6H4NO6P
Mol. Mass.:
217.0739
SMILES:
[O-][N+](=O)c1ccc(O[P+]([O-])([O-])[O-])cc1