Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Transient receptor potential cation channel subfamily V member 1
Ligand
BDBM20334
Substrate
BDBM10852
Meas. Tech.
Ca2+ Influx Functional Assay for the Determination of in Vitro Activity.
pH
7.4±n/a
Temperature
295.15±n/a K
EC50
4±3 nM
Comments
The pharmacokinetic profile of the compound in rats was characterized by low plasma clearance (CLp = 0.6 L/(h kg)), reasonable oral bioavailability (F = 46%), but a low volume of distribution (Vbeta = 0.6 L/kg) and a short plasma elimination half-life (T1/2 = 0.6 h, iv).
Citation
 Gomtsyan, A; Bayburt, EK; Schmidt, RG; Zheng, GZ; Perner, RJ; Didomenico, S; Koenig, JR; Turner, S; Jinkerson, T; Drizin, I; Hannick, SM; Macri, BS; McDonald, HA; Honore, P; Wismer, CT; Marsh, KC; Wetter, J; Stewart, KD; Oie, T; Jarvis, MF; Surowy, CS; Faltynek, CR; Lee, CH Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure-activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J Med Chem 48:744-52 (2005) [PubMed] Article
Gomtsyan, A; Bayburt, EK; Schmidt, RG; Zheng, GZ; Perner, RJ; Didomenico, S; Koenig, JR; Turner, S; Jinkerson, T; Drizin, I; Hannick, SM; Macri, BS; McDonald, HA; Honore, P; Wismer, CT; Marsh, KC; Wetter, J; Stewart, KD; Oie, T; Jarvis, MF; Surowy, CS; Faltynek, CR; Lee, CH Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure-activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J Med Chem 48:744-52 (2005) [PubMed] Article More Info.:
Target
Name:
Transient receptor potential cation channel subfamily V member 1
Synonyms:
Capsaicin receptor | OTRPC1 | Osm-9-like TRP channel 1 | TRPV1 | TRPV1_HUMAN | Transient receptor potential cation channel subfamily V member 1 | Transient receptor potential cation channel subfamily V member 1 (TRPV1) | Transient receptor potential cation channel subfamily V member 1 (VR1/TRPV1) | Transient receptor potential cation channel subfamily V member 1(TRPV1) | VR1 | Vanilloid VR1 | Vanilloid receptor | Vanilloid receptor 1 | Vanilloid receptor 1 (TrpV1/Vr1) | Vanilloid receptor 1 (VRI/TRPV1)
Type:
Protein
Mol. Mass.:
94960.75
Organism:
Homo sapiens (Human)
Description:
Q8NER1
Residue:
839
Sequence:
MKKWSSTDLGAAADPLQKDTCPDPLDGDPNSRPPPAKPQLSTAKSRTRLFGKGDSEEAFPVDCPHEEGELDSCPTITVSPVITIQRPGDGPTGARLLSQDSVAASTEKTLRLYDRRSIFEAVAQNNCQDLESLLLFLQKSKKHLTDNEFKDPETGKTCLLKAMLNLHDGQNTTIPLLLEIARQTDSLKELVNASYTDSYYKGQTALHIAIERRNMALVTLLVENGADVQAAAHGDFFKKTKGRPGFYFGELPLSLAACTNQLGIVKFLLQNSWQTADISARDSVGNTVLHALVEVADNTADNTKFVTSMYNEILMLGAKLHPTLKLEELTNKKGMTPLALAAGTGKIGVLAYILQREIQEPECRHLSRKFTEWAYGPVHSSLYDLSCIDTCEKNSVLEVIAYSSSETPNRHDMLLVEPLNRLLQDKWDRFVKRIFYFNFLVYCLYMIIFTMAAYYRPVDGLPPFKMEKTGDYFRVTGEILSVLGGVYFFFRGIQYFLQRRPSMKTLFVDSYSEMLFFLQSLFMLATVVLYFSHLKEYVASMVFSLALGWTNMLYYTRGFQQMGIYAVMIEKMILRDLCRFMFVYIVFLFGFSTAVVTLIEDGKNDSLPSESTSHRWRGPACRPPDSSYNSLYSTCLELFKFTIGMGDLEFTENYDFKAVFIILLLAYVILTYILLLNMLIALMGETVNKIAQESKNIWKLQRAITILDTEKSFLKCMRKAFRSGKLLQVGYTPDGKDDYRWCFRVDEVNWTTWNTNVGIINEDPGNCEGVKRTLSFSLRSSRVSGRHWKNFALVPLLREASARDRQSAQPEEVYLRQFSGSLKPEDAEVFKSPAASGEK
Inhibitor
Name:
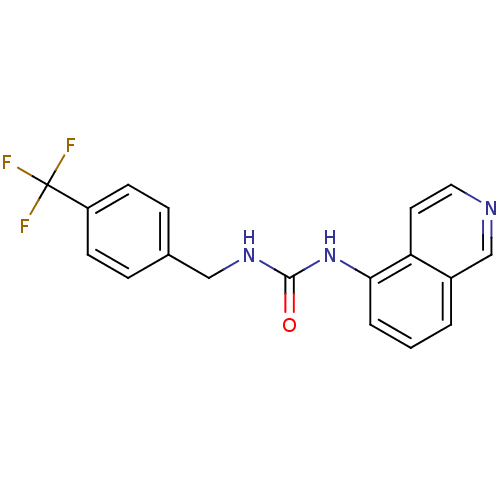
BDBM20334
Synonyms:
1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea | 1-isoquinolin-5-yl-3-{[4-(trifluoromethyl)phenyl]methyl}urea | A-425619 | CHEMBL104028 | JMC48744 Compound 14a
Type:
Small organic molecule
Emp. Form.:
C18H14F3N3O
Mol. Mass.:
345.3185
SMILES:
FC(F)(F)c1ccc(CNC(=O)Nc2cccc3cnccc23)cc1
Substrate