Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Purine nucleoside phosphorylase
Ligand
BDBM22103
Substrate
BDBM22104
Meas. Tech.
PNP Inhibition Assay
pH
7.7±n/a
Temperature
295.15±n/a K
Ki
0.229±0.015 nM
Km
40000±n/a nM
Citation
 Evans, GB; Furneaux, RH; Greatrex, B; Murkin, AS; Schramm, VL; Tyler, PC Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J Med Chem 51:948-56 (2008) [PubMed] Article
Evans, GB; Furneaux, RH; Greatrex, B; Murkin, AS; Schramm, VL; Tyler, PC Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J Med Chem 51:948-56 (2008) [PubMed] Article More Info.:
Target
Name:
Purine nucleoside phosphorylase
Synonyms:
Inosine phosphorylase | Inosine-guanosine phosphorylase | NP | PNP | PNPH_HUMAN | Purine nucleoside phosphorylase (PNPase)
Type:
Enzyme
Mol. Mass.:
32119.53
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
289
Sequence:
MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRSTVPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGLNPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQMGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSLITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS
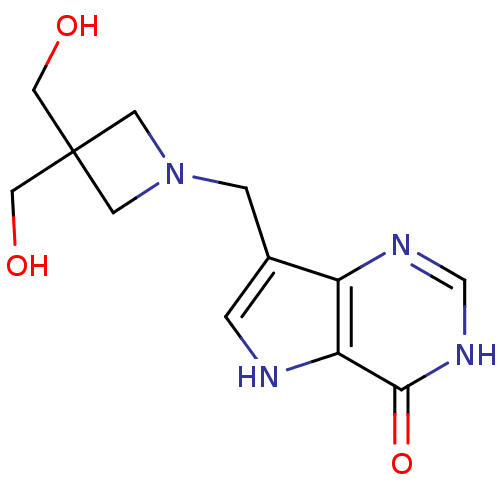
Inhibitor
Name:
BDBM22103
Synonyms:
7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5H-pyrrolo[3,2-d]pyrimidin-4-one | Azetidine based compound, 34
Type:
Small organic molecule
Emp. Form.:
C12H16N4O3
Mol. Mass.:
264.2804
SMILES:
OCC1(CO)CN(Cc2c[nH]c3c2nc[nH]c3=O)C1
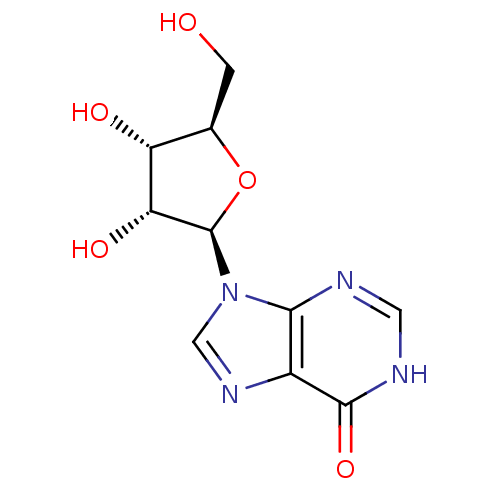
Substrate
Name:
BDBM22104
Synonyms:
9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-3H-purin-6-one | Atorel | Hypoxanthosine | Inosine | cid_6021
Type:
Purine Nucleoside
Emp. Form.:
C10H12N4O5
Mol. Mass.:
268.2261
SMILES:
OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c1nc[nH]c2=O