Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Epithelial discoidin domain-containing receptor 1
Ligand
BDBM13533
Substrate
biotinylated affinity ligand
Meas. Tech.
Kinase Inhibitor Selectivity Profiling Assay
pH
7.4±n/a
Temperature
298.15±n/a K
Kd
1.9±n/a nM
Citation
More Info.:
Target
Name:
Epithelial discoidin domain-containing receptor 1
Synonyms:
CAK | DDR1 | DDR1_HUMAN | Discoidin domain receptor 1 (DDR1) | Discoidin domain-containing receptor 1 (DDR1) | Discoidin receptor tyrosine kinase | EDDR1 | Epithelial discoidin domain receptor 1 | Epithelial discoidin domain receptor 1 (DDR1) | Epithelial discoidin domain-containing receptor 1 | Epithelial discoidin domain-containing receptor 1 (DDR1) | HGK2 | Mammary carcinoma kinase 10 | NEP | NTRK4 | PTK3A | Protein-tyrosine kinase RTK 6 | RTK6 | TRKE | Tyrosine-protein kinase CAK
Type:
Tyrosine-protein kinase
Mol. Mass.:
101130.02
Organism:
Homo sapiens (Human)
Description:
Q08345
Residue:
913
Sequence:
MGPEALSSLLLLLLVASGDADMKGHFDPAKCRYALGMQDRTIPDSDISASSSWSDSTAARHSRLESSDGDGAWCPAGSVFPKEEEYLQVDLQRLHLVALVGTQGRHAGGLGKEFSRSYRLRYSRDGRRWMGWKDRWGQEVISGNEDPEGVVLKDLGPPMVARLVRFYPRADRVMSVCLRVELYGCLWRDGLLSYTAPVGQTMYLSEAVYLNDSTYDGHTVGGLQYGGLGQLADGVVGLDDFRKSQELRVWPGYDYVGWSNHSFSSGYVEMEFEFDRLRAFQAMQVHCNNMHTLGARLPGGVECRFRRGPAMAWEGEPMRHNLGGNLGDPRARAVSVPLGGRVARFLQCRFLFAGPWLLFSEISFISDVVNNSSPALGGTFPPAPWWPPGPPPTNFSSLELEPRGQQPVAKAEGSPTAILIGCLVAIILLLLLIIALMLWRLHWRRLLSKAERRVLEEELTVHLSVPGDTILINNRPGPREPPPYQEPRPRGNPPHSAPCVPNGSALLLSNPAYRLLLATYARPPRGPGPPTPAWAKPTNTQAYSGDYMEPEKPGAPLLPPPPQNSVPHYAEADIVTLQGVTGGNTYAVPALPPGAVGDGPPRVDFPRSRLRFKEKLGEGQFGEVHLCEVDSPQDLVSLDFPLNVRKGHPLLVAVKILRPDATKNARNDFLKEVKIMSRLKDPNIIRLLGVCVQDDPLCMITDYMENGDLNQFLSAHQLEDKAAEGAPGDGQAAQGPTISYPMLLHVAAQIASGMRYLATLNFVHRDLATRNCLVGENFTIKIADFGMSRNLYAGDYYRVQGRAVLPIRWMAWECILMGKFTTASDVWAFGVTLWEVLMLCRAQPFGQLTDEQVIENAGEFFRDQGRQVYLSRPPACPQGLYELMLRCWSRESEQRPPFSQLHRFLAEDALNTV
Inhibitor
Name:
BDBM13533
Synonyms:
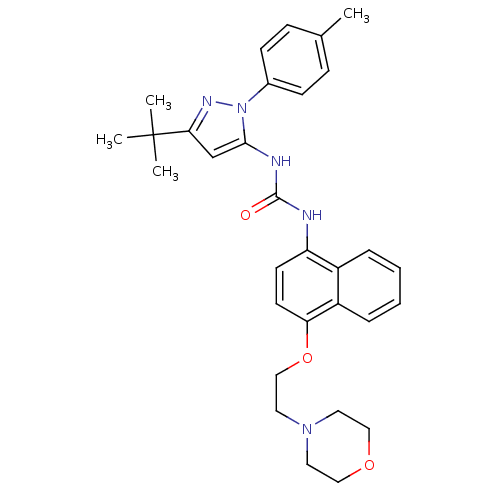
1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3-[4-(2-morpholin-4-ylethoxy)naphthalen-1-yl]urea | 1-[5-tert-butyl-2-(4-methylphenyl)-3-pyrazolyl]-3-[4-[2-(4-morpholinyl)ethoxy]-1-naphthalenyl]urea | 1-[5-tert-butyl-2-(4-methylphenyl)pyrazol-3-yl]-3-[4-(2-morpholin-4-ylethoxy)naphthalen-1-yl]urea | 1-[5-tert-butyl-2-(p-tolyl)pyrazol-3-yl]-3-[4-(2-morpholinoethoxy)-1-naphthyl]urea | 3-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-1-[4-(2-morpholin-4-ylethoxy)naphthalen-1-yl]urea | 3-[3-tert-butyl-1-(4-methylphenyl)-1H-pyrazol-5-yl]-1-{4-[2-(morpholin-4-yl)ethoxy]naphthalen-1-yl}urea | BIRB 796 | BIRB-796 | BIRB-796, 3 | CHEMBL103667 | D3RKN_73 | Doramapimod | US11407771, Compound 43 | US8933228, BIRB 796 | US9187470, 43 (BIRB-796) | US9242960, BIRB 796 | US9260410, BIRB796 | cid_156422 | diaryl urea compound 10
Type:
Small organic molecule
Emp. Form.:
C31H37N5O3
Mol. Mass.:
527.6572
SMILES:
Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C
Substrate
Name:
biotinylated affinity ligand
Synonyms:
n/a
Type:
n/a
Mol. Mass.:
358.43
Organism:
n/a
Description:
n/a
Residue:
3
Sequence:
NA