Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serotonin 2 (5-HT2) receptor
Ligand
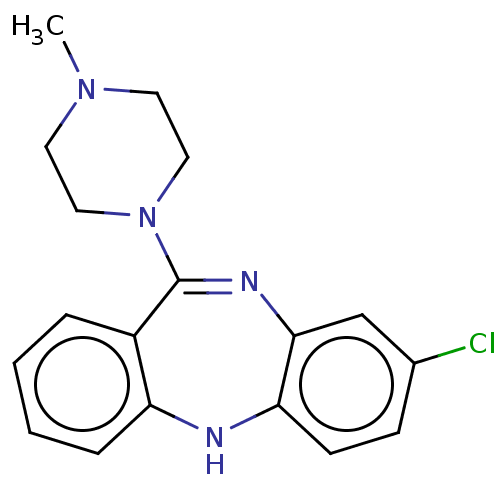
BDBM50001884
Substrate
n/a
Meas. Tech.
ChEMBL_1913 (CHEMBL616968)
IC50
7.8±n/a nM
Citation
 Perregaard, J; Arnt, J; Bøgesø, KP; Hyttel, J; Sánchez, C Noncataleptogenic, centrally acting dopamine D-2 and serotonin 5-HT2 antagonists within a series of 3-substituted 1-(4-fluorophenyl)-1H-indoles. J Med Chem 35:1092-101 (1992) [PubMed] Article
Perregaard, J; Arnt, J; Bøgesø, KP; Hyttel, J; Sánchez, C Noncataleptogenic, centrally acting dopamine D-2 and serotonin 5-HT2 antagonists within a series of 3-substituted 1-(4-fluorophenyl)-1H-indoles. J Med Chem 35:1092-101 (1992) [PubMed] Article More Info.:
Target
Name:
Serotonin 2 (5-HT2) receptor
Synonyms:
5-hydroxytryptamine receptor 2A/2B/2C
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of ChEMBL is 2197419
Components:
This complex has 3 components.
Component 1
Name:
5-hydroxytryptamine receptor 2B
Synonyms:
5-HT2B | 5-hydroxytryptamine receptor 2B | 5HT2B_RAT | Htr2b | Serotonin 2b (5-HT2b) receptor | Serotonin receptor 2a and 2b (5HT2A and 5HT2B) | Srl
Type:
Enzyme Catalytic Domain
Mol. Mass.:
53668.47
Organism:
RAT
Description:
5-HT2B HTR2B RAT::P30994
Residue:
479
Sequence:
MASSYKMSEQSTISEHILQKTCDHLILTDRSGLKAESAAEEMKQTAENQGNTVHWAALLIFAVIIPTIGGNILVILAVSLEKRLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEATWPLPLALCPAWLFLDVLFSTASIMHLCAISLDRYIAIKKPIQANQCNSRTTAFVKITVVWLISIGIAIPVPIKGIEADVVNAHNITCELTKDRFGSFMLFGSLAAFFAPLTIMIVTYFLTIHALRKKAYLVRNRPPQRLTRWTVSTVLQREDSSFSSPEKMVMLDGSHKDKILPNSTDETLMRRMSSAGKKPAQTISNEQRASKVLGIVFLFFLLMWCPFFITNVTLALCDSCNQTTLKTLLQIFVWVGYVSSGVNPLIYTLFNKTFREAFGRYITCNYQATKSVKVLRKCSSTLYFGNSMVENSKFFTKHGIRNGINPAMYQSPVRLRSSTIQSSSIILLNTFLTENDGDKVEDQVSYI
Component 2
Name:
5-hydroxytryptamine receptor 2C
Synonyms:
5-HT2C | 5-hydroxytryptamine receptor 2C | 5-hydroxytryptamine receptor 2C (5HT2c) | 5HT2C_RAT | 5ht1c | Htr1c | Htr2c | Serotonin (5-HT) receptor | Serotonin receptor 2a and 2c (5HT2A and 5HT2C)
Type:
Enzyme
Mol. Mass.:
51935.10
Organism:
Rattus norvegicus (Rat)
Description:
P08909
Residue:
460
Sequence:
MVNLGNAVRSLLMHLIGLLVWQFDISISPVAAIVTDTFNSSDGGRLFQFPDGVQNWPALSIVVIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVWPLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWAISIGVSVPIPVIGLRDESKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYFLTIYVLRRQTLMLLRGHTEEELANMSLNFLNCCCKKNGGEEENAPNPNPDQKPRRKKKEKRPRGTMQAINNEKKASKVLGIVFFVFLIMWCPFFITNILSVLCGKACNQKLMEKLLNVFVWIGYVCSGINPLVYTLFNKIYRRAFSKYLRCDYKPDKKPPVRQIPRVAATALSGRELNVNIYRHTNERVARKANDPEPGIEMQVENLELPVNPSNVVSERISSV
Component 3
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2A | 5-HT2 | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_RAT | Htr2 | Htr2a | Serotonin Receptor 2A
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
52852.05
Organism:
Rattus norvegicus (rat)
Description:
Rat cortex membranes 5-HT2A receptors.
Residue:
471
Sequence:
MEILCEDNISLSSIPNSLMQLGDGPRLYHNDFNSRDANTSEASNWTIDAENRTNLSCEGYLPPTCLSILHLQEKNWSALLTTVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAIWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVAFFIPLTIMVITYFLTIKSLQKEATLCVSDLSTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYAGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNENVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENRKPLQLILVNTIPALAYKSSQLQVGQKKNSQEDAEQTVDDCSMVTLGKQQSEENCTDNIETVNEKVSCV
Inhibitor
Name:
BDBM50001884
Synonyms:
2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl-2,3-dihydro-indol-1-yl)-ethanone | 3-Hydroxy-2-phenyl-propionic acid 8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl ester | 3-chloro-6-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine | 8-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine (Clozapine) | 8-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine( Clozepine ) | 8-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine(Ciozapine) | 8-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine(Clopazine) | 8-chloro-11-(4-methyl-piperazin-1-yl)-10H-dibenzo[b,e][1,4]diazepine | 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine | CHEMBL42 | CHEMBL538973 | CLOZAPINE | CLOZAPINE, 8-CHLORO-11-(4-METHYL-PIPERAZIN-1-YL)-5H-DIBENZO[B,E][1,4]DIAZEPINE | CLOZARIL | HF 1854 | US10167256, Clozapine | US10752588, Compound Clozapine | US11498896, Compound Clozapine
Type:
Small organic molecule
Emp. Form.:
C18H19ClN4
Mol. Mass.:
326.823
SMILES:
CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8|