Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine protease 1
Ligand
BDBM50071693
Substrate
n/a
Meas. Tech.
ChEBML_212708
IC50
1271±n/a nM
Citation
 Owens, TD; Semple, JE Alkoxide-catalyzed ring-opening of a novel homosaccharin derivative: synthesis of potent, selective P3-lactam thrombin inhibitors containing P4-o-alkoxycarbonylbenzylsulfonamide residues. Bioorg Med Chem Lett 8:3683-8 (1999) [PubMed] Article
Owens, TD; Semple, JE Alkoxide-catalyzed ring-opening of a novel homosaccharin derivative: synthesis of potent, selective P3-lactam thrombin inhibitors containing P4-o-alkoxycarbonylbenzylsulfonamide residues. Bioorg Med Chem Lett 8:3683-8 (1999) [PubMed] Article More Info.:
Target
Name:
Serine protease 1
Synonyms:
Alpha-trypsin chain 1 | Alpha-trypsin chain 2 | Beta-trypsin | Cationic trypsinogen | PRSS1 | Serine protease 1 | TRP1 | TRY1 | TRY1_HUMAN | TRYP1 | Thrombin & trypsin | Trypsin | Trypsin I | Trypsin-1
Type:
Enzyme
Mol. Mass.:
26557.80
Organism:
Homo sapiens (Human)
Description:
P07477
Residue:
247
Sequence:
MNPLLILTFVAAALAAPFDDDDKIVGGYNCEENSVPYQVSLNSGYHFCGGSLINEQWVVSAGHCYKSRIQVRLGEHNIEVLEGNEQFINAAKIIRHPQYDRKTLNNDIMLIKLSSRAVINARVSTISLPTAPPATGTKCLISGWGNTASSGADYPDELQCLDAPVLSQAKCEASYPGKITSNMFCVGFLEGGKDSCQGDSGGPVVCNGQLQGVVSWGDGCAQKNKPGVYTKVYNYVKWIKNTIAANS
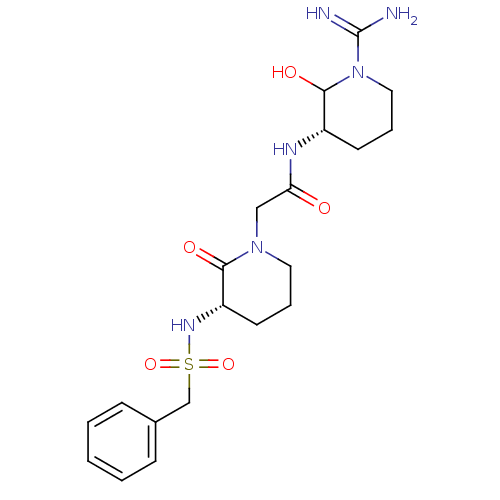
Inhibitor
Name:
BDBM50071693
Synonyms:
CHEMBL38927 | CVS-1578 | N-((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-yl)-2-((S)-2-oxo-3-phenylmethanesulfonylamino-piperidin-1-yl)-acetamide | N-(1-Carbamimidoyl-2-hydroxy-piperidin-3-yl)-2-(2-oxo-3-phenylmethanesulfonylamino-piperidin-1-yl)-acetamide
Type:
Small organic molecule
Emp. Form.:
C20H30N6O5S
Mol. Mass.:
466.554
SMILES:
NC(=N)N1CCC[C@H](NC(=O)CN2CCC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O