Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase ABL1
Ligand
BDBM15244
Substrate
n/a
Meas. Tech.
ChEMBL_325109 (CHEMBL859871)
Kd
1200±n/a nM
Citation
 Fabian, MA; Biggs, WH; Treiber, DK; Atteridge, CE; Azimioara, MD; Benedetti, MG; Carter, TA; Ciceri, P; Edeen, PT; Floyd, M; Ford, JM; Galvin, M; Gerlach, JL; Grotzfeld, RM; Herrgard, S; Insko, DE; Insko, MA; Lai, AG; Lélias, JM; Mehta, SA; Milanov, ZV; Velasco, AM; Wodicka, LM; Patel, HK; Zarrinkar, PP; Lockhart, DJ A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23:329-36 (2005) [PubMed] Article
Fabian, MA; Biggs, WH; Treiber, DK; Atteridge, CE; Azimioara, MD; Benedetti, MG; Carter, TA; Ciceri, P; Edeen, PT; Floyd, M; Ford, JM; Galvin, M; Gerlach, JL; Grotzfeld, RM; Herrgard, S; Insko, DE; Insko, MA; Lai, AG; Lélias, JM; Mehta, SA; Milanov, ZV; Velasco, AM; Wodicka, LM; Patel, HK; Zarrinkar, PP; Lockhart, DJ A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23:329-36 (2005) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein kinase ABL1
Synonyms:
ABL | ABL1 | ABL1_HUMAN | Abelson murine leukemia viral oncogene homolog 1 | JTK7 | Proto-oncogene c-Abl | Proto-oncogene tyrosine-protein kinase ABL1 | Tyrosine-protein kinase (ABL) | Tyrosine-protein kinase ABL | Tyrosine-protein kinase ABL1 (ABL) | V-abl Abelson murine leukemia viral oncogene homolog 1 | c-ABL | p150 | tyrosine-protein kinase ABL1 isoform a
Type:
Enzyme
Mol. Mass.:
122897.30
Organism:
Homo sapiens (Human)
Description:
P00519
Residue:
1130
Sequence:
MLEICLKLVGCKSKKGLSSSSSCYLEEALQRPVASDFEPQGLSEAARWNSKENLLAGPSENDPNLFVALYDFVASGDNTLSITKGEKLRVLGYNHNGEWCEAQTKNGQGWVPSNYITPVNSLEKHSWYHGPVSRNAAEYLLSSGINGSFLVRESESSPGQRSISLRYEGRVYHYRINTASDGKLYVSSESRFNTLAELVHHHSTVADGLITTLHYPAPKRNKPTVYGVSPNYDKWEMERTDITMKHKLGGGQYGEVYEGVWKKYSLTVAVKTLKEDTMEVEEFLKEAAVMKEIKHPNLVQLLGVCTREPPFYIITEFMTYGNLLDYLRECNRQEVNAVVLLYMATQISSAMEYLEKKNFIHRDLAARNCLVGENHLVKVADFGLSRLMTGDTYTAHAGAKFPIKWTAPESLAYNKFSIKSDVWAFGVLLWEIATYGMSPYPGIDLSQVYELLEKDYRMERPEGCPEKVYELMRACWQWNPSDRPSFAEIHQAFETMFQESSISDEVEKELGKQGVRGAVSTLLQAPELPTKTRTSRRAAEHRDTTDVPEMPHSKGQGESDPLDHEPAVSPLLPRKERGPPEGGLNEDERLLPKDKKTNLFSALIKKKKKTAPTPPKRSSSFREMDGQPERRGAGEEEGRDISNGALAFTPLDTADPAKSPKPSNGAGVPNGALRESGGSGFRSPHLWKKSSTLTSSRLATGEEEGGGSSSKRFLRSCSASCVPHGAKDTEWRSVTLPRDLQSTGRQFDSSTFGGHKSEKPALPRKRAGENRSDQVTRGTVTPPPRLVKKNEEAADEVFKDIMESSPGSSPPNLTPKPLRRQVTVAPASGLPHKEEAGKGSALGTPAAAEPVTPTSKAGSGAPGGTSKGPAEESRVRRHKHSSESPGRDKGKLSRLKPAPPPPPAASAGKAGGKPSQSPSQEAAGEAVLGAKTKATSLVDAVNSDAAKPSQPGEGLKKPVLPATPKPQSAKPSGTPISPAPVPSTLPSASSALAGDQPSSTAFIPLISTRVSLRKTRQPPERIASGAITKGVVLDSTEALCLAISRNSEQMASHSAVLEAGKNLYTFCVSYVDSIQQMRNKFAFREAINKLENNLRELQICPATAGSGPAATQDFSKLLSSVKEISDIVQR
Inhibitor
Name:
BDBM15244
Synonyms:
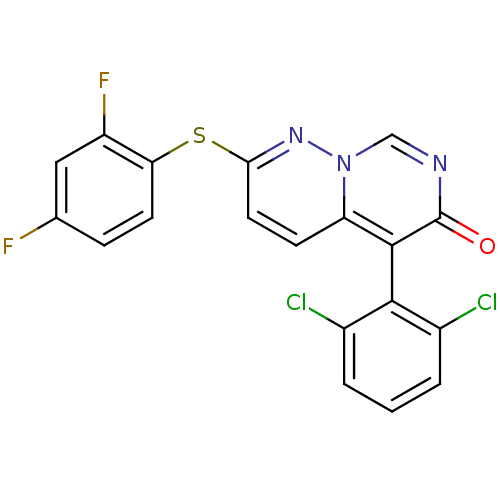
5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyrimido[1,6-b]pyridazin-6-one | 5-(2,6-dichlorophenyl)-2-[(2,4-difluorophenyl)sulfanyl]-6H-pyrimido[1,6-a]pyridazin-6-one | 5-(2,6-dichlorophenyl)-2-[(2,4-difluorophenyl)thio]-6-pyrimido[1,6-b]pyridazinone | 5-(2,6-dichlorophenyl)-2-[(2,4-difluorophenyl)thio]pyrimido[1,6-b]pyridazin-6-one | 5-(2,6-dichlorophenyl)-9-(2,4-difluorophenyl)sulfanyl-1,3,10-triazabicyclo[4.4.0]deca-2,5,7,9-tetraen-4-one | 5-[2,6-bis(chloranyl)phenyl]-2-[2,4-bis(fluoranyl)phenyl]sulfanyl-pyrimido[1,6-b]pyridazin-6-one | VX-745 | VX745 | cid_3038525
Type:
Small organic molecule
Emp. Form.:
C19H9Cl2F2N3OS
Mol. Mass.:
436.262
SMILES:
Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)|