Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Telomerase reverse transcriptase
Ligand
BDBM50180763
Substrate
n/a
Meas. Tech.
ChEMBL_327150 (CHEMBL864040)
EC50
255±n/a nM
Citation
 Moore, MJ; Schultes, CM; Cuesta, J; Cuenca, F; Gunaratnam, M; Tanious, FA; Wilson, WD; Neidle, S Trisubstituted acridines as G-quadruplex telomere targeting agents. Effects of extensions of the 3,6- and 9-side chains on quadruplex binding, telomerase activity, and cell proliferation. J Med Chem 49:582-99 (2006) [PubMed] Article
Moore, MJ; Schultes, CM; Cuesta, J; Cuenca, F; Gunaratnam, M; Tanious, FA; Wilson, WD; Neidle, S Trisubstituted acridines as G-quadruplex telomere targeting agents. Effects of extensions of the 3,6- and 9-side chains on quadruplex binding, telomerase activity, and cell proliferation. J Med Chem 49:582-99 (2006) [PubMed] Article More Info.:
Target
Name:
Telomerase reverse transcriptase
Synonyms:
EST2 | TCS1 | TERT | TERT_HUMAN | TRT
Type:
PROTEIN
Mol. Mass.:
127099.03
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1447029
Residue:
1132
Sequence:
MPRAPRCRAVRSLLRSHYREVLPLATFVRRLGPQGWRLVQRGDPAAFRALVAQCLVCVPWDARPPPAAPSFRQVSCLKELVARVLQRLCERGAKNVLAFGFALLDGARGGPPEAFTTSVRSYLPNTVTDALRGSGAWGLLLRRVGDDVLVHLLARCALFVLVAPSCAYQVCGPPLYQLGAATQARPPPHASGPRRRLGCERAWNHSVREAGVPLGLPAPGARRRGGSASRSLPLPKRPRRGAAPEPERTPVGQGSWAHPGRTRGPSDRGFCVVSPARPAEEATSLEGALSGTRHSHPSVGRQHHAGPPSTSRPPRPWDTPCPPVYAETKHFLYSSGDKEQLRPSFLLSSLRPSLTGARRLVETIFLGSRPWMPGTPRRLPRLPQRYWQMRPLFLELLGNHAQCPYGVLLKTHCPLRAAVTPAAGVCAREKPQGSVAAPEEEDTDPRRLVQLLRQHSSPWQVYGFVRACLRRLVPPGLWGSRHNERRFLRNTKKFISLGKHAKLSLQELTWKMSVRDCAWLRRSPGVGCVPAAEHRLREEILAKFLHWLMSVYVVELLRSFFYVTETTFQKNRLFFYRKSVWSKLQSIGIRQHLKRVQLRELSEAEVRQHREARPALLTSRLRFIPKPDGLRPIVNMDYVVGARTFRREKRAERLTSRVKALFSVLNYERARRPGLLGASVLGLDDIHRAWRTFVLRVRAQDPPPELYFVKVDVTGAYDTIPQDRLTEVIASIIKPQNTYCVRRYAVVQKAAHGHVRKAFKSHVSTLTDLQPYMRQFVAHLQETSPLRDAVVIEQSSSLNEASSGLFDVFLRFMCHHAVRIRGKSYVQCQGIPQGSILSTLLCSLCYGDMENKLFAGIRRDGLLLRLVDDFLLVTPHLTHAKTFLRTLVRGVPEYGCVVNLRKTVVNFPVEDEALGGTAFVQMPAHGLFPWCGLLLDTRTLEVQSDYSSYARTSIRASLTFNRGFKAGRNMRRKLFGVLRLKCHSLFLDLQVNSLQTVCTNIYKILLLQAYRFHACVLQLPFHQQVWKNPTFFLRVISDTASLCYSILKAKNAGMSLGAKGAAGPLPSEAVQWLCHQAFLLKLTRHRVTYVPLLGSLRTAQTQLSRKLPGTTLTALEAAANPALPSDFKTILD
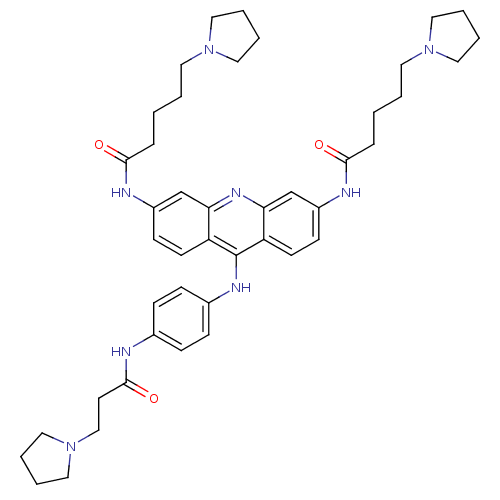
Inhibitor
Name:
BDBM50180763
Synonyms:
3,6-bis[5-(pyrrolidin-1-yl)pentanamido]-9-{4'-[3''-(pyrrolidin-1-yl)propanamido]anilino}acridine | CHEMBL202717
Type:
Small organic molecule
Emp. Form.:
C44H58N8O3
Mol. Mass.:
746.9831
SMILES:
O=C(CCCCN1CCCC1)Nc1ccc2c(Nc3ccc(NC(=O)CCN4CCCC4)cc3)c3ccc(NC(=O)CCCCN4CCCC4)cc3nc2c1