Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Melanin-concentrating hormone receptor 1
Ligand
BDBM50184935
Substrate
n/a
Meas. Tech.
ChEMBL_390360 (CHEMBL869840)
Ki
2±n/a nM
Citation
 McBriar, MD; Guzik, H; Shapiro, S; Xu, R; Paruchova, J; Clader, JW; O'neill, K; Hawes, B; Sorota, S; Margulis, M; Tucker, K; Weston, DJ; Cox, K Bicyclo[3.1.0]hexyl urea melanin concentrating hormone (MCH) receptor-1 antagonists: impacting hERG liability via aryl modifications. Bioorg Med Chem Lett 16:4262-5 (2006) [PubMed] Article
McBriar, MD; Guzik, H; Shapiro, S; Xu, R; Paruchova, J; Clader, JW; O'neill, K; Hawes, B; Sorota, S; Margulis, M; Tucker, K; Weston, DJ; Cox, K Bicyclo[3.1.0]hexyl urea melanin concentrating hormone (MCH) receptor-1 antagonists: impacting hERG liability via aryl modifications. Bioorg Med Chem Lett 16:4262-5 (2006) [PubMed] Article More Info.:
Target
Name:
Melanin-concentrating hormone receptor 1
Synonyms:
G-protein coupled receptor 24 | GPR24 | MCH receptor 1 | MCH-1R | MCH-R1 | MCHR | MCHR-1 | MCHR1 | MCHR1_HUMAN | Melanin Concentrating Hormone 1 | Melanin-Concentrating Hormone Receptor 1 (MCH1R) | Melanin-concentrating hormone receptor | Melanin-concentrating hormone receptor 1 (MCH-1) | Melanin-concentrating hormone receptor 1 (MCH1) | Melanin-concentrating hormone receptor 1 (MCHR-1) | Melanin-concentrating hormone receptor 1 (MCHR1) | SLC-1 | SLC1 | Somatostatin receptor-like protein
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
45976.27
Organism:
Homo sapiens (Human)
Description:
Membranes from CHO-K1 cells stably expressing human MCH1R were used in assays.
Residue:
422
Sequence:
MSVGAMKKGVGRAVGLGGGSGCQATEEDPLPNCGACAPGQGGRRWRLPQPAWVEGSSARLWEQATGTGWMDLEASLLPTGPNASNTSDGPDNLTSAGSPPRTGSISYINIIMPSVFGTICLLGIIGNSTVIFAVVKKSKLHWCNNVPDIFIINLSVVDLLFLLGMPFMIHQLMGNGVWHFGETMCTLITAMDANSQFTSTYILTAMAIDRYLATVHPISSTKFRKPSVATLVICLLWALSFISITPVWLYARLIPFPGGAVGCGIRLPNPDTDLYWFTLYQFFLAFALPFVVITAAYVRILQRMTSSVAPASQRSIRLRTKRVTRTAIAICLVFFVCWAPYYVLQLTQLSISRPTLTFVYLYNAAISLGYANSCLNPFVYIVLCETFRKRLVLSVKPAAQGQLRAVSNAQTADEERTESKGT
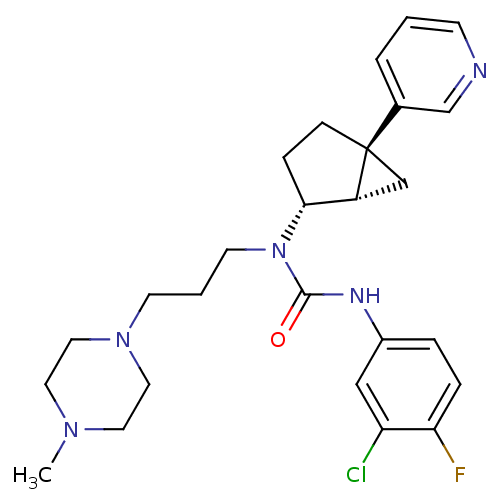
Inhibitor
Name:
BDBM50184935
Synonyms:
3-(3-chloro-4-fluorophenyl)-1-(3-(4-methylpiperazin-1-yl)propyl)-1-((1S,2R,5S)-5-(pyridin-3-yl)bicyclo[3.1.0]hexan-2-yl)urea | CHEMBL425410 | N'-(3-chloro-4-fluorophenyl)-N-[3-(4-methyl-1-piperazinyl)-propyl]-N-[trans-5-(3-pyridinyl)bicyclo[3.1.0]hex-2-yl]urea
Type:
Small organic molecule
Emp. Form.:
C26H33ClFN5O
Mol. Mass.:
486.025
SMILES:
CN1CCN(CCCN([C@@H]2CC[C@@]3(C[C@H]23)c2cccnc2)C(=O)Nc2ccc(F)c(Cl)c2)CC1