Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cathepsin S
Ligand
BDBM50221221
Substrate
n/a
Meas. Tech.
ChEMBL_449532 (CHEMBL899800)
IC50
27±n/a nM
Citation
 Wei, J; Pio, BA; Cai, H; Meduna, SP; Sun, S; Gu, Y; Jiang, W; Thurmond, RL; Karlsson, L; Edwards, JP Pyrazole-based cathepsin S inhibitors with improved cellular potency. Bioorg Med Chem Lett 17:5525-8 (2007) [PubMed] Article
Wei, J; Pio, BA; Cai, H; Meduna, SP; Sun, S; Gu, Y; Jiang, W; Thurmond, RL; Karlsson, L; Edwards, JP Pyrazole-based cathepsin S inhibitors with improved cellular potency. Bioorg Med Chem Lett 17:5525-8 (2007) [PubMed] Article More Info.:
Target
Name:
Cathepsin S
Synonyms:
CATS_HUMAN | CTSS | Cathepsin S (Cat S) | cathepsin S preproprotein
Type:
Protein
Mol. Mass.:
37507.38
Organism:
Homo sapiens (Human)
Description:
P25774
Residue:
331
Sequence:
MKRLVCVLLVCSSAVAQLHKDPTLDHHWHLWKKTYGKQYKEKNEEAVRRLIWEKNLKFVMLHNLEHSMGMHSYDLGMNHLGDMTSEEVMSLMSSLRVPSQWQRNITYKSNPNRILPDSVDWREKGCVTEVKYQGSCGACWAFSAVGALEAQLKLKTGKLVSLSAQNLVDCSTEKYGNKGCNGGFMTTAFQYIIDNKGIDSDASYPYKAMDQKCQYDSKYRAATCSKYTELPYGREDVLKEAVANKGPVSVGVDARHPSFFLYRSGVYYEPSCTQNVNHGVLVVGYGDLNGKEYWLVKNSWGHNFGEEGYIRMARNKGNHCGIASFPSYPEI
Inhibitor
Name:
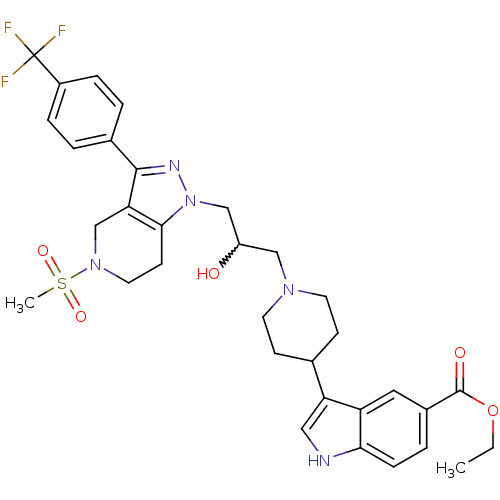
BDBM50221221
Synonyms:
CHEMBL397622 | ethyl 3-(1-(2-hydroxy-3-(5-(methylsulfonyl)-3-(4-(trifluoromethyl)phenyl)-4,5,6,7-tetrahydropyrazolo[4,3-c]pyridin-1-yl)propyl)piperidin-4-yl)-1H-indole-5-carboxylate
Type:
Small organic molecule
Emp. Form.:
C33H38F3N5O5S
Mol. Mass.:
673.746
SMILES:
CCOC(=O)c1ccc2[nH]cc(C3CCN(CC(O)Cn4nc(c5CN(CCc45)S(C)(=O)=O)-c4ccc(cc4)C(F)(F)F)CC3)c2c1 |w:17.17|