Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Telomerase reverse transcriptase
Ligand
BDBM50253719
Substrate
n/a
Meas. Tech.
ChEMBL_512488 (CHEMBL966978)
EC50
15000±n/a nM
Citation
 Zagotto, G; Sissi, C; Lucatello, L; Pivetta, C; Cadamuro, SA; Fox, KR; Neidle, S; Palumbo, M Aminoacyl-anthraquinone conjugates as telomerase inhibitors: synthesis, biophysical and biological evaluation. J Med Chem 51:5566-74 (2008) [PubMed] Article
Zagotto, G; Sissi, C; Lucatello, L; Pivetta, C; Cadamuro, SA; Fox, KR; Neidle, S; Palumbo, M Aminoacyl-anthraquinone conjugates as telomerase inhibitors: synthesis, biophysical and biological evaluation. J Med Chem 51:5566-74 (2008) [PubMed] Article More Info.:
Target
Name:
Telomerase reverse transcriptase
Synonyms:
EST2 | TCS1 | TERT | TERT_HUMAN | TRT
Type:
PROTEIN
Mol. Mass.:
127099.03
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1447029
Residue:
1132
Sequence:
MPRAPRCRAVRSLLRSHYREVLPLATFVRRLGPQGWRLVQRGDPAAFRALVAQCLVCVPWDARPPPAAPSFRQVSCLKELVARVLQRLCERGAKNVLAFGFALLDGARGGPPEAFTTSVRSYLPNTVTDALRGSGAWGLLLRRVGDDVLVHLLARCALFVLVAPSCAYQVCGPPLYQLGAATQARPPPHASGPRRRLGCERAWNHSVREAGVPLGLPAPGARRRGGSASRSLPLPKRPRRGAAPEPERTPVGQGSWAHPGRTRGPSDRGFCVVSPARPAEEATSLEGALSGTRHSHPSVGRQHHAGPPSTSRPPRPWDTPCPPVYAETKHFLYSSGDKEQLRPSFLLSSLRPSLTGARRLVETIFLGSRPWMPGTPRRLPRLPQRYWQMRPLFLELLGNHAQCPYGVLLKTHCPLRAAVTPAAGVCAREKPQGSVAAPEEEDTDPRRLVQLLRQHSSPWQVYGFVRACLRRLVPPGLWGSRHNERRFLRNTKKFISLGKHAKLSLQELTWKMSVRDCAWLRRSPGVGCVPAAEHRLREEILAKFLHWLMSVYVVELLRSFFYVTETTFQKNRLFFYRKSVWSKLQSIGIRQHLKRVQLRELSEAEVRQHREARPALLTSRLRFIPKPDGLRPIVNMDYVVGARTFRREKRAERLTSRVKALFSVLNYERARRPGLLGASVLGLDDIHRAWRTFVLRVRAQDPPPELYFVKVDVTGAYDTIPQDRLTEVIASIIKPQNTYCVRRYAVVQKAAHGHVRKAFKSHVSTLTDLQPYMRQFVAHLQETSPLRDAVVIEQSSSLNEASSGLFDVFLRFMCHHAVRIRGKSYVQCQGIPQGSILSTLLCSLCYGDMENKLFAGIRRDGLLLRLVDDFLLVTPHLTHAKTFLRTLVRGVPEYGCVVNLRKTVVNFPVEDEALGGTAFVQMPAHGLFPWCGLLLDTRTLEVQSDYSSYARTSIRASLTFNRGFKAGRNMRRKLFGVLRLKCHSLFLDLQVNSLQTVCTNIYKILLLQAYRFHACVLQLPFHQQVWKNPTFFLRVISDTASLCYSILKAKNAGMSLGAKGAAGPLPSEAVQWLCHQAFLLKLTRHRVTYVPLLGSLRTAQTQLSRKLPGTTLTALEAAANPALPSDFKTILD
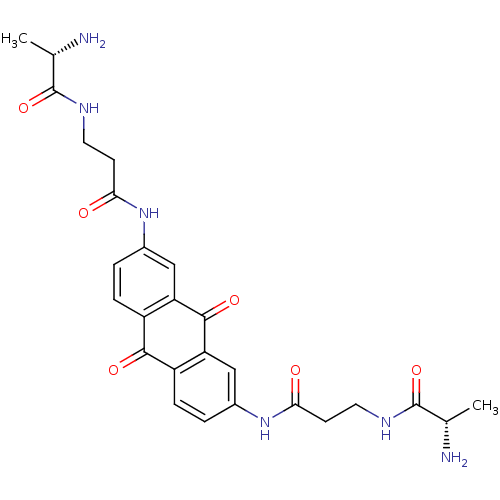
Inhibitor
Name:
BDBM50253719
Synonyms:
(S)-2-Amino-N-(2-{7-[3-((S)-2-amino-propionylamino)-propionylamino]-9,10-dioxo-9,10-dihydro-anthracen-2-ylcarbamoyl}-ethyl)-propionamide | CHEMBL462255
Type:
Small organic molecule
Emp. Form.:
C26H30N6O6
Mol. Mass.:
522.553
SMILES:
C[C@H](N)C(=O)NCCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCNC(=O)[C@H](C)N)cc3C(=O)c2c1 |r|