Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mitogen-activated protein kinase kinase kinase kinase 5
Ligand
BDBM13534
Substrate
n/a
Meas. Tech.
ChEMBL_537086 (CHEMBL988918)
Kd
53±n/a nM
Citation
 Bamborough, P; Drewry, D; Harper, G; Smith, GK; Schneider, K Assessment of chemical coverage of kinome space and its implications for kinase drug discovery. J Med Chem 51:7898-914 (2008) [PubMed] Article
Bamborough, P; Drewry, D; Harper, G; Smith, GK; Schneider, K Assessment of chemical coverage of kinome space and its implications for kinase drug discovery. J Med Chem 51:7898-914 (2008) [PubMed] Article More Info.:
Target
Name:
Mitogen-activated protein kinase kinase kinase kinase 5
Synonyms:
2.7.11.1 | KHS | Kinase homologous to SPS1/STE20 | M4K5_HUMAN | MAP4K5 | MAPK/ERK kinase kinase kinase 5 | MEK kinase kinase 5 | MEKKK 5
Type:
PROTEIN
Mol. Mass.:
95039.51
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1495741
Residue:
846
Sequence:
MEAPLRPAADILRRNPQQDYELVQRVGSGTYGDVYKARNVHTGELAAVKIIKLEPGDDFSLIQQEIFMVKECKHCNIVAYFGSYLSREKLWICMEYCGGGSLQDIYHVTGPLSELQIAYVCRETLQGLAYLHTKGKMHRDIKGANILLTDHGDVKLADFGVAAKITATIAKRKSFIGTPYWMAPEVAAVEKNGGYNQLCDIWAVGITAIELGELQPPMFDLHPMRALFLMSKSNFQPPKLKDKTKWSSTFHNFVKIALTKNPKKRPTAERLLTHTFVAQPGLSRALAVELLDKVNNPDNHAHYTEADDDDFEPHAIIRHTIRSTNRNARAERTASEINFDKLQFEPPLRKETEARDEMGLSSDPNFMLQWNPFVDGANTGKSTSKRAIPPPLPPKPRISSYPEDNFPDEEKASTIKHCPDSESRAPQILRRQSSPSCGPVAETSSIGNGDGISKLMSENTEGSAQAPQLPRKKDKRDFPKPAINGLPPTPKVLMGACFSKVFDGCPLKINCATSWIHPDTKDQYIIFGTEDGIYTLNLNELHEATMEQLFPRKCTWLYVINNTLMSLSVGKTFQLYSHNLIALFEHAKKPGLAAHIQTHRFPDRILPRKFALTTKIPDTKGCHKCCIVRNPYTGHKYLCGALQSGIVLLQWYEPMQKFMLIKHFDFPLPSPLNVFEMLVIPEQEYPMVCVAISKGTESNQVVQFETINLNSASSWFTEIGAGSQQLDSIHVTQLERDTVLVCLDKFVKIVNLQGKLKSSKKLASELSFDFRIESVVCLQDSVLAFWKHGMQGKSFKSDEVTQEISDETRVFRLLGSDRVVVLESRPTENPTAHSNLYILAGHENSY
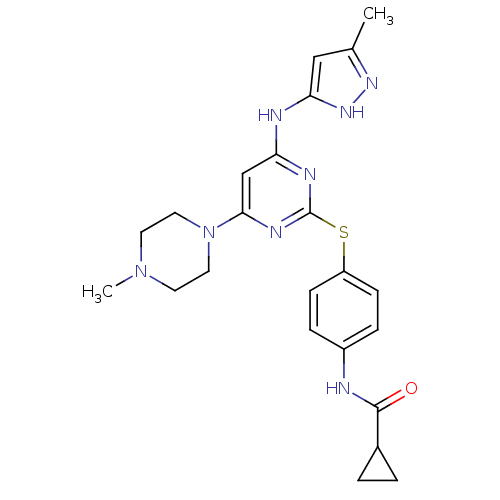
Inhibitor
Name:
BDBM13534
Synonyms:
CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl)amino]-6-(4-methylpiperazin-1-yl)pyrimidin-2-yl}sulfanyl)phenyl]cyclopropanecarboxamide | N-[4-[[4-(4-methylpiperazino)-6-[(5-methyl-1H-pyrazol-3-yl)amino]pyrimidin-2-yl]thio]phenyl]cyclopropanecarboxamide | VX-680 | VX680 | cyclopropane carboxylic acid {4-[4-(4-methyl-piperazin-1-yl)-6-(5-methyl-2H-pyrazol-3-ylamino)-pyrimidin-2ylsulphanyl]-phenyl}-amide
Type:
Small organic molecule
Emp. Form.:
C23H28N8OS
Mol. Mass.:
464.586
SMILES:
CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1