Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Polyunsaturated fatty acid 5-lipoxygenase
Ligand
BDBM17638
Substrate
n/a
Meas. Tech.
ChEBML_4175
IC50
>100000±n/a nM
Citation
 Kramer, JB; Boschelli, DH; Connor, DT; Kostlan, CR; Flynn, DL; Dyer, RD; Bornemeier, DA; Kennedy, JA; Wright, CD; Kuipers, PJ Synthesis of reversed hydroxamic acids of indomethacin: dual inhibitors of cyclooxygenase and 5-lipoxygenase Bioorg Med Chem Lett 2:1655-1660 (1992) Article
Kramer, JB; Boschelli, DH; Connor, DT; Kostlan, CR; Flynn, DL; Dyer, RD; Bornemeier, DA; Kennedy, JA; Wright, CD; Kuipers, PJ Synthesis of reversed hydroxamic acids of indomethacin: dual inhibitors of cyclooxygenase and 5-lipoxygenase Bioorg Med Chem Lett 2:1655-1660 (1992) Article More Info.:
Target
Name:
Polyunsaturated fatty acid 5-lipoxygenase
Synonyms:
Alox5 | Arachidonate 5-lipoxygenase | LOX5_RAT
Type:
PROTEIN
Mol. Mass.:
78082.31
Organism:
Rattus norvegicus
Description:
ChEMBL_1432947
Residue:
673
Sequence:
MPSYTVTVATGSQWFAGTDDYIYLSLIGSAGCSEKHLLDKAFYNDFERGGRDSYDVTVDEELGEIYLVKIEKRKYRLHDDWYLKYITLKTPHDYIEFPCYRWITGEGEIVLRDGCAKLARDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVLNYSKAMENLFINRFMHMFQSSWHDFADFEKIFVKISNTISERVKNHWQEDLMFGYQFLNGCNPVLIKRCTELPKKLPVTTEMVECSLERQLSLEQEVQEGNIFIVDYELLDGIDANKTDPCTHQFLAAPICLLYKNLANKIVPIAIQLNQTPGEKNPIFLPTDSKYDWLLAKIWVRSSDFHIHQTITHLLRTHLVSEVFGIAMYRQLPAVHPLFKLLVAHVRFTIAINTKAREQLNCEYGLFDKANATGGGGHVQMVQRAVQDLTYSSLCFPEAIKARGMDNTEDIPYYFYRDDGLLVWEAIQSFTTEVVSIYYEDDQVVEEDQELQDFVKDVYVYGMRGRKASGFPKSIKSREKLSEYLTVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCWHLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMIRFRKNLEAIVSVIAERNKNKKLPYYYLSPDRIPNSVAI
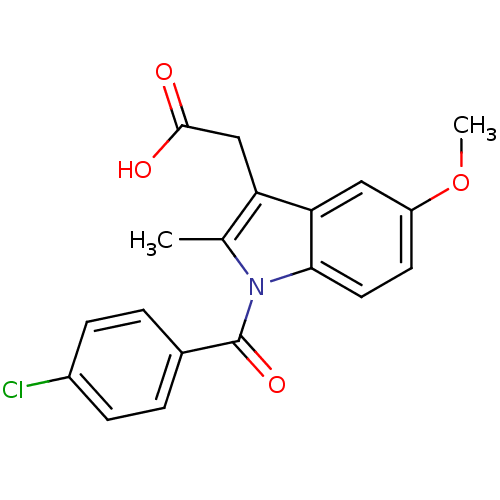
Inhibitor
Name:
BDBM17638
Synonyms:
2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid | CHEMBL6 | Indocin | Indomethacin | US11478464, Compound Indomethacin | US11786535, Compound Indomethacin | US9271961, Indomethacin | indometacin
Type:
Small organic molecule
Emp. Form.:
C19H16ClNO4
Mol. Mass.:
357.788
SMILES:
COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1