Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nociceptin receptor
Ligand
BDBM50296842
Substrate
n/a
Meas. Tech.
ChEMBL_582620 (CHEMBL1054183)
EC50
1.3±n/a nM
Citation
 Nishimura, H; Li, J; Isozaki, K; Okada, K; Matsushima, A; Nose, T; Costa, T; Shimohigashi, Y Discriminatory synergistic effect of Trp-substitutions in superagonist [(Arg/Lys)(14), (Arg/Lys)(15)]nociceptin on ORL1 receptor binding and activation. Bioorg Med Chem 17:5683-7 (2009) [PubMed] Article
Nishimura, H; Li, J; Isozaki, K; Okada, K; Matsushima, A; Nose, T; Costa, T; Shimohigashi, Y Discriminatory synergistic effect of Trp-substitutions in superagonist [(Arg/Lys)(14), (Arg/Lys)(15)]nociceptin on ORL1 receptor binding and activation. Bioorg Med Chem 17:5683-7 (2009) [PubMed] Article More Info.:
Target
Name:
Nociceptin receptor
Synonyms:
Nociceptin/Orphanin FQ, NOP receptor | Nociceptin/mu opioid receptor | OPRX_RAT | Oor | Oprl | Oprl1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
40531.08
Organism:
RAT
Description:
Nociceptin/Orphanin FQ, NOP receptor 0 RAT::P35370
Residue:
367
Sequence:
MESLFPAPYWEVLYGSHFQGNLSLLNETVPHHLLLNASHSAFLPLGLKVTIVGLYLAVCIGGLLGNCLVMYVILRHTKMKTATNIYIFNLALADTLVLLTLPFQGTDILLGFWPFGNALCKTVIAIDYYNMFTSTFTLTAMSVDRYVAICHPIRALDVRTSSKAQAVNVAIWALASVVGVPVAIMGSAQVEDEEIECLVEIPAPQDYWGPVFAICIFLFSFIIPVLIISVCYSLMIRRLRGVRLLSGSREKDRNLRRITRLVLVVVAVFVGCWTPVQVFVLVQGLGVQPGSETAVAILRFCTALGYVNSCLNPILYAFLDENFKACFRKFCCASSLHREMQVSDRVRSIAKDVGLGCKTSETVPRPA
Inhibitor
Name:
BDBM50296842
Synonyms:
CHEMBL559130 | FGGFTGARKSARKWKNQ
Type:
Small organic molecule
Emp. Form.:
C87H135N29O22
Mol. Mass.:
1939.1839
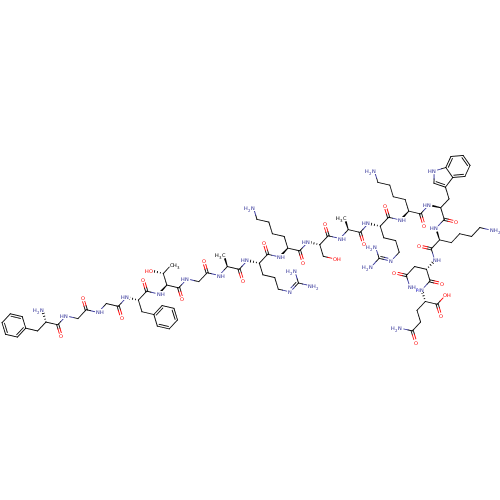
SMILES:
C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:1.1,73.75,89.90,42.44,26.27,112.115,129.132,58.59,3.3,wD:78.79,7.15,47.48,98.99,121.124,67.68,(8.85,-33.5,;10.18,-34.27,;11.52,-33.52,;10.17,-35.81,;8.84,-36.58,;7.5,-35.8,;7.51,-34.26,;6.17,-36.57,;6.16,-38.11,;7.49,-38.88,;7.47,-40.42,;8.8,-41.2,;10.14,-40.43,;10.14,-38.88,;8.83,-38.12,;4.84,-35.78,;3.5,-36.55,;3.5,-38.09,;2.17,-35.77,;.83,-36.54,;-.5,-35.75,;-.49,-34.21,;-1.84,-36.52,;-3.17,-35.74,;-4.51,-36.51,;-4.51,-38.05,;-5.83,-35.72,;-7.17,-36.49,;-5.82,-34.18,;-7.15,-33.41,;-7.14,-31.87,;-8.47,-31.09,;-9.81,-31.85,;-9.82,-33.4,;-8.48,-34.17,;11.5,-36.6,;11.5,-38.14,;12.84,-35.83,;14.17,-36.61,;15.51,-35.85,;15.51,-34.31,;16.84,-36.63,;18.18,-35.86,;18.18,-34.32,;19.51,-36.64,;19.5,-38.18,;20.84,-35.88,;22.18,-36.66,;22.17,-38.2,;20.83,-38.96,;20.82,-40.5,;19.48,-41.26,;19.47,-42.8,;18.13,-43.56,;20.8,-43.58,;23.51,-35.89,;23.52,-34.35,;24.84,-36.67,;26.18,-35.91,;26.19,-34.37,;27.52,-33.61,;27.53,-32.07,;28.87,-31.3,;28.88,-29.76,;27.51,-36.69,;27.5,-38.23,;28.85,-35.92,;30.18,-36.7,;30.17,-38.24,;28.83,-39,;31.52,-35.94,;31.52,-34.4,;32.85,-36.72,;34.18,-35.95,;34.19,-34.41,;35.51,-36.73,;35.5,-38.27,;36.85,-35.97,;38.18,-36.75,;38.17,-38.29,;36.83,-39.05,;36.82,-40.59,;35.48,-41.35,;35.47,-42.89,;34.14,-43.65,;36.8,-43.67,;39.52,-35.98,;39.52,-34.44,;40.85,-36.76,;42.19,-36,;42.19,-34.46,;43.53,-33.7,;43.54,-32.16,;44.88,-31.39,;44.89,-29.85,;43.52,-36.78,;43.51,-38.32,;44.86,-36.01,;46.18,-36.79,;46.17,-38.33,;47.5,-39.11,;48.91,-38.48,;49.93,-39.63,;49.16,-40.96,;49.63,-42.41,;48.61,-43.54,;47.1,-43.22,;46.63,-41.76,;47.66,-40.63,;47.52,-36.03,;47.53,-34.49,;48.85,-36.81,;50.19,-36.04,;50.2,-34.5,;51.53,-33.74,;51.54,-32.21,;52.87,-31.45,;52.88,-29.92,;51.52,-36.82,;51.51,-38.36,;52.86,-36.06,;54.19,-36.84,;54.18,-38.38,;55.51,-39.16,;56.84,-38.39,;55.5,-40.7,;55.53,-36.08,;55.53,-34.54,;56.85,-36.85,;58.19,-36.1,;58.2,-34.56,;59.54,-33.79,;59.54,-32.25,;58.21,-31.47,;60.88,-31.49,;59.52,-36.87,;60.86,-36.11,;59.51,-38.41,)|