Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Telomerase reverse transcriptase
Ligand
BDBM50134030
Substrate
n/a
Meas. Tech.
ChEMBL_588495 (CHEMBL1041235)
IC50
90±n/a nM
Citation
 Laronze-Cochard, M; Kim, YM; Brassart, B; Riou, JF; Laronze, JY; Sapi, J Synthesis and biological evaluation of novel 4,5-bis(dialkylaminoalkyl)-substituted acridines as potent telomeric G-quadruplex ligands. Eur J Med Chem 44:3880-8 (2009) [PubMed] Article
Laronze-Cochard, M; Kim, YM; Brassart, B; Riou, JF; Laronze, JY; Sapi, J Synthesis and biological evaluation of novel 4,5-bis(dialkylaminoalkyl)-substituted acridines as potent telomeric G-quadruplex ligands. Eur J Med Chem 44:3880-8 (2009) [PubMed] Article More Info.:
Target
Name:
Telomerase reverse transcriptase
Synonyms:
EST2 | TCS1 | TERT | TERT_HUMAN | TRT
Type:
PROTEIN
Mol. Mass.:
127099.03
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1447029
Residue:
1132
Sequence:
MPRAPRCRAVRSLLRSHYREVLPLATFVRRLGPQGWRLVQRGDPAAFRALVAQCLVCVPWDARPPPAAPSFRQVSCLKELVARVLQRLCERGAKNVLAFGFALLDGARGGPPEAFTTSVRSYLPNTVTDALRGSGAWGLLLRRVGDDVLVHLLARCALFVLVAPSCAYQVCGPPLYQLGAATQARPPPHASGPRRRLGCERAWNHSVREAGVPLGLPAPGARRRGGSASRSLPLPKRPRRGAAPEPERTPVGQGSWAHPGRTRGPSDRGFCVVSPARPAEEATSLEGALSGTRHSHPSVGRQHHAGPPSTSRPPRPWDTPCPPVYAETKHFLYSSGDKEQLRPSFLLSSLRPSLTGARRLVETIFLGSRPWMPGTPRRLPRLPQRYWQMRPLFLELLGNHAQCPYGVLLKTHCPLRAAVTPAAGVCAREKPQGSVAAPEEEDTDPRRLVQLLRQHSSPWQVYGFVRACLRRLVPPGLWGSRHNERRFLRNTKKFISLGKHAKLSLQELTWKMSVRDCAWLRRSPGVGCVPAAEHRLREEILAKFLHWLMSVYVVELLRSFFYVTETTFQKNRLFFYRKSVWSKLQSIGIRQHLKRVQLRELSEAEVRQHREARPALLTSRLRFIPKPDGLRPIVNMDYVVGARTFRREKRAERLTSRVKALFSVLNYERARRPGLLGASVLGLDDIHRAWRTFVLRVRAQDPPPELYFVKVDVTGAYDTIPQDRLTEVIASIIKPQNTYCVRRYAVVQKAAHGHVRKAFKSHVSTLTDLQPYMRQFVAHLQETSPLRDAVVIEQSSSLNEASSGLFDVFLRFMCHHAVRIRGKSYVQCQGIPQGSILSTLLCSLCYGDMENKLFAGIRRDGLLLRLVDDFLLVTPHLTHAKTFLRTLVRGVPEYGCVVNLRKTVVNFPVEDEALGGTAFVQMPAHGLFPWCGLLLDTRTLEVQSDYSSYARTSIRASLTFNRGFKAGRNMRRKLFGVLRLKCHSLFLDLQVNSLQTVCTNIYKILLLQAYRFHACVLQLPFHQQVWKNPTFFLRVISDTASLCYSILKAKNAGMSLGAKGAAGPLPSEAVQWLCHQAFLLKLTRHRVTYVPLLGSLRTAQTQLSRKLPGTTLTALEAAANPALPSDFKTILD
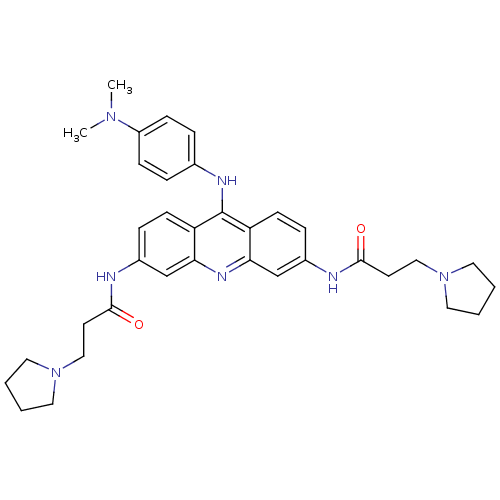
Inhibitor
Name:
BDBM50134030
Synonyms:
9-[4-(N,N-dimethylamino)phenylamino]-3,6-bis(3-pyrrolodinopropionamido) acridine | CHEMBL336434 | N,N'-(9-{[4-(dimethylamino)phenyl]amino}acridine-3,6-diyl)bis(3-pyrrolidin-1-ylpropanamide)
Type:
Small organic molecule
Emp. Form.:
C35H43N7O2
Mol. Mass.:
593.7616
SMILES:
CN(C)c1ccc(Nc2c3ccc(NC(=O)CCN4CCCC4)cc3nc3cc(NC(=O)CCN4CCCC4)ccc23)cc1