Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha
Ligand
BDBM50300983
Substrate
n/a
Meas. Tech.
ChEMBL_597960 (CHEMBL1042745)
IC50
15±n/a nM
Citation
 Hughes, RO; Walker, JK; Rogier, DJ; Heasley, SE; Blevis-Bal, RM; Benson, AG; Jacobsen, EJ; Cubbage, JW; Fobian, YM; Owen, DR; Freskos, JN; Molyneaux, JM; Brown, DL; Acker, BA; Maddux, TM; Tollefson, MB; Moon, JB; Mischke, BV; Rumsey, JM; Zheng, Y; MacInnes, A; Bond, BR; Yu, Y Optimization of the aminopyridopyrazinones class of PDE5 inhibitors: discovery of 3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one. Bioorg Med Chem Lett 19:5209-13 (2009) [PubMed] Article
Hughes, RO; Walker, JK; Rogier, DJ; Heasley, SE; Blevis-Bal, RM; Benson, AG; Jacobsen, EJ; Cubbage, JW; Fobian, YM; Owen, DR; Freskos, JN; Molyneaux, JM; Brown, DL; Acker, BA; Maddux, TM; Tollefson, MB; Moon, JB; Mischke, BV; Rumsey, JM; Zheng, Y; MacInnes, A; Bond, BR; Yu, Y Optimization of the aminopyridopyrazinones class of PDE5 inhibitors: discovery of 3-[(trans-4-hydroxycyclohexyl)amino]-7-(6-methoxypyridin-3-yl)-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one. Bioorg Med Chem Lett 19:5209-13 (2009) [PubMed] Article More Info.:
Target
Name:
Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha
Synonyms:
PDE6A | PDE6A_HUMAN | PDEA | Rod cGMP-specific 3',5'-cyclic phosphodiesterase alpha
Type:
PROTEIN
Mol. Mass.:
99531.10
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1347866
Residue:
860
Sequence:
MGEVTAEEVEKFLDSNIGFAKQYYNLHYRAKLISDLLGAKEAAVDFSNYHSPSSMEESEIIFDLLRDFQENLQTEKCIFNVMKKLCFLLQADRMSLFMYRTRNGIAELATRLFNVHKDAVLEDCLVMPDQEIVFPLDMGIVGHVAHSKKIANVPNTEEDEHFCDFVDILTEYKTKNILASPIMNGKDVVAIIMAVNKVDGSHFTKRDEEILLKYLNFANLIMKVYHLSYLHNCETRRGQILLWSGSKVFEELTDIERQFHKALYTVRAFLNCDRYSVGLLDMTKQKEFFDVWPVLMGEVPPYSGPRTPDGREINFYKVIDYILHGKEDIKVIPNPPPDHWALVSGLPAYVAQNGLICNIMNAPAEDFFAFQKEPLDESGWMIKNVLSMPIVNKKEEIVGVATFYNRKDGKPFDEMDETLMESLTQFLGWSVLNPDTYESMNKLENRKDIFQDIVKYHVKCDNEEIQKILKTREVYGKEPWECEEEELAEILQAELPDADKYEINKFHFSDLPLTELELVKCGIQMYYELKVVDKFHIPQEALVRFMYSLSKGYRKITYHNWRHGFNVGQTMFSLLVTGKLKRYFTDLEALAMVTAAFCHDIDHRGTNNLYQMKSQNPLAKLHGSSILERHHLEFGKTLLRDESLNIFQNLNRRQHEHAIHMMDIAIIATDLALYFKKRTMFQKIVDQSKTYESEQEWTQYMMLEQTRKEIVMAMMMTACDLSAITKPWEVQSQVALLVAAEFWEQGDLERTVLQQNPIPMMDRNKADELPKLQVGFIDFVCTFVYKEFSRFHEEITPMLDGITNNRKEWKALADEYDAKMKVQEEKKQKQQSAKSAAAGNQPGGNPSPGGATTSKSCCIQ
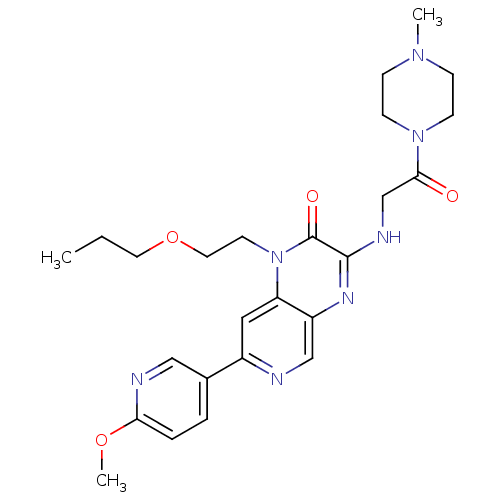
Inhibitor
Name:
BDBM50300983
Synonyms:
7-(6-methoxypyridin-3-yl)-3-(2-(4-methylpiperazin-1-yl)-2-oxoethylamino)-1-(2-propoxyethyl)pyrido[3,4-b]pyrazin-2(1H)-one | CHEMBL585918
Type:
Small organic molecule
Emp. Form.:
C25H33N7O4
Mol. Mass.:
495.574
SMILES:
CCCOCCn1c2cc(ncc2nc(NCC(=O)N2CCN(C)CC2)c1=O)-c1ccc(OC)nc1