Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
72 kDa type IV collagenase
Ligand
BDBM50305842
Substrate
n/a
Meas. Tech.
ChEMBL_605226 (CHEMBL1069306)
Ki
>25000±n/a nM
Citation
 Schnute, ME; O'Brien, PM; Nahra, J; Morris, M; Howard Roark, W; Hanau, CE; Ruminski, PG; Scholten, JA; Fletcher, TR; Hamper, BC; Carroll, JN; Patt, WC; Shieh, HS; Collins, B; Pavlovsky, AG; Palmquist, KE; Aston, KW; Hitchcock, J; Rogers, MD; McDonald, J; Johnson, AR; Munie, GE; Wittwer, AJ; Man, CF; Settle, SL; Nemirovskiy, O; Vickery, LE; Agawal, A; Dyer, RD; Sunyer, T Discovery of (pyridin-4-yl)-2H-tetrazole as a novel scaffold to identify highly selective matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. Bioorg Med Chem Lett 20:576-80 (2010) [PubMed] Article
Schnute, ME; O'Brien, PM; Nahra, J; Morris, M; Howard Roark, W; Hanau, CE; Ruminski, PG; Scholten, JA; Fletcher, TR; Hamper, BC; Carroll, JN; Patt, WC; Shieh, HS; Collins, B; Pavlovsky, AG; Palmquist, KE; Aston, KW; Hitchcock, J; Rogers, MD; McDonald, J; Johnson, AR; Munie, GE; Wittwer, AJ; Man, CF; Settle, SL; Nemirovskiy, O; Vickery, LE; Agawal, A; Dyer, RD; Sunyer, T Discovery of (pyridin-4-yl)-2H-tetrazole as a novel scaffold to identify highly selective matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. Bioorg Med Chem Lett 20:576-80 (2010) [PubMed] Article More Info.:
Target
Name:
72 kDa type IV collagenase
Synonyms:
72 kDa gelatinase | 72 kDa type IV collagenase precursor | CLG4A | Gelatinase A | Gelatinase A (MMP-2) | MMP2 | MMP2_HUMAN | Matrix metalloproteinase-2 | Matrix metalloproteinase-2 (MMP 2) | Matrix metalloproteinase-2 (MMP2) | Matrix metalloproteinases 2 (MMP-2) | TBE-1
Type:
Enzyme
Mol. Mass.:
73870.36
Organism:
Homo sapiens (Human)
Description:
P08253
Residue:
660
Sequence:
MEALMARGALTGPLRALCLLGCLLSHAAAAPSPIIKFPGDVAPKTDKELAVQYLNTFYGCPKESCNLFVLKDTLKKMQKFFGLPQTGDLDQNTIETMRKPRCGNPDVANYNFFPRKPKWDKNQITYRIIGYTPDLDPETVDDAFARAFQVWSDVTPLRFSRIHDGEADIMINFGRWEHGDGYPFDGKDGLLAHAFAPGTGVGGDSHFDDDELWTLGEGQVVRVKYGNADGEYCKFPFLFNGKEYNSCTDTGRSDGFLWCSTTYNFEKDGKYGFCPHEALFTMGGNAEGQPCKFPFRFQGTSYDSCTTEGRTDGYRWCGTTEDYDRDKKYGFCPETAMSTVGGNSEGAPCVFPFTFLGNKYESCTSAGRSDGKMWCATTANYDDDRKWGFCPDQGYSLFLVAAHEFGHAMGLEHSQDPGALMAPIYTYTKNFRLSQDDIKGIQELYGASPDIDLGTGPTPTLGPVTPEICKQDIVFDGIAQIRGEIFFFKDRFIWRTVTPRDKPMGPLLVATFWPELPEKIDAVYEAPQEEKAVFFAGNEYWIYSASTLERGYPKPLTSLGLPPDVQRVDAAFNWSKNKKTYIFAGDKFWRYNEVKKKMDPGFPKLIADAWNAIPDNLDAVVDLQGGGHSYFFKGAYYLKLENQSLKSVKFGSIKSDWLGC
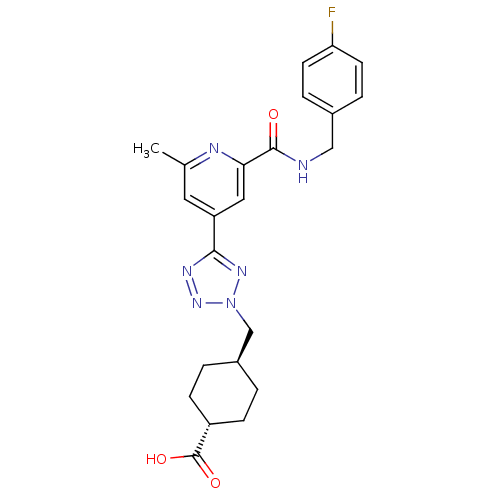
Inhibitor
Name:
BDBM50305842
Synonyms:
CHEMBL603656 | trans-4-((5-(2-(4-fluorobenzylcarbamoyl)-6-methylpyridin-4-yl)-2H-tetrazol-2-yl)methyl)cyclohexanecarboxylic acid
Type:
Small organic molecule
Emp. Form.:
C23H25FN6O3
Mol. Mass.:
452.4814
SMILES:
Cc1cc(cc(n1)C(=O)NCc1ccc(F)cc1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:26.31,wD:23.24,(30.11,-12.24,;29.32,-10.92,;30.07,-9.57,;29.28,-8.26,;27.74,-8.27,;26.98,-9.62,;27.77,-10.94,;25.44,-9.64,;24.69,-10.98,;24.66,-8.31,;23.12,-8.33,;22.33,-7.01,;23.08,-5.67,;22.3,-4.34,;20.75,-4.36,;19.97,-3.04,;20,-5.72,;20.79,-7.03,;30.03,-6.91,;29.39,-5.51,;30.52,-4.46,;31.87,-5.21,;33.27,-4.57,;34.61,-5.32,;34.63,-6.85,;35.98,-7.6,;37.3,-6.81,;37.27,-5.26,;35.93,-4.52,;38.65,-7.56,;38.68,-9.1,;39.97,-6.77,;31.57,-6.73,)|