Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate receptor 1
Ligand
BDBM50002369
Substrate
n/a
Meas. Tech.
ChEMBL_627982 (CHEMBL1105503)
EC50
60000±n/a nM
Citation
 Fleming, JJ; England, PM Developing a complete pharmacology for AMPA receptors: a perspective on subtype-selective ligands. Bioorg Med Chem 18:1381-7 (2010) [PubMed] Article
Fleming, JJ; England, PM Developing a complete pharmacology for AMPA receptors: a perspective on subtype-selective ligands. Bioorg Med Chem 18:1381-7 (2010) [PubMed] Article More Info.:
Target
Name:
Glutamate receptor 1
Synonyms:
AMPA-selective glutamate receptor 1 | GLUH1 | GLUR1 | GRIA1 | GRIA1_HUMAN | GluR-1 | GluR-A | GluR-K1 | Glutamate AMPA 1 | Glutamate receptor 1 | Glutamate receptor AMPA 1/2 | Glutamate receptor ionotropic AMPA | Glutamate receptor ionotropic, AMPA 1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
101512.92
Organism:
Homo sapiens (Human)
Description:
Glutamate AMPA 1 GRIA1 HUMAN::P42261
Residue:
906
Sequence:
MQHIFAFFCTGFLGAVVGANFPNNIQIGGLFPNQQSQEHAAFRFALSQLTEPPKLLPQIDIVNISDSFEMTYRFCSQFSKGVYAIFGFYERRTVNMLTSFCGALHVCFITPSFPVDTSNQFVLQLRPELQDALISIIDHYKWQKFVYIYDADRGLSVLQKVLDTAAEKNWQVTAVNILTTTEEGYRMLFQDLEKKKERLVVVDCESERLNAILGQIIKLEKNGIGYHYILANLGFMDIDLNKFKESGANVTGFQLVNYTDTIPAKIMQQWKNSDARDHTRVDWKRPKYTSALTYDGVKVMAEAFQSLRRQRIDISRRGNAGDCLANPAVPWGQGIDIQRALQQVRFEGLTGNVQFNEKGRRTNYTLHVIEMKHDGIRKIGYWNEDDKFVPAATDAQAGGDNSSVQNRTYIVTTILEDPYVMLKKNANQFEGNDRYEGYCVELAAEIAKHVGYSYRLEIVSDGKYGARDPDTKAWNGMVGELVYGRADVAVAPLTITLVREEVIDFSKPFMSLGISIMIKKPQKSKPGVFSFLDPLAYEIWMCIVFAYIGVSVVLFLVSRFSPYEWHSEEFEEGRDQTTSDQSNEFGIFNSLWFSLGAFMQQGCDISPRSLSGRIVGGVWWFFTLIIISSYTANLAAFLTVERMVSPIESAEDLAKQTEIAYGTLEAGSTKEFFRRSKIAVFEKMWTYMKSAEPSVFVRTTEEGMIRVRKSKGKYAYLLESTMNEYIEQRKPCDTMKVGGNLDSKGYGIATPKGSALRNPVNLAVLKLNEQGLLDKLKNKWWYDKGECGSGGGDSKDKTSALSLSNVAGVFYILIGGLGLAMLVALIEFCYKSRSESKRMKGFCLIPQQSINEAIRTSTLPRNSGAGASSGGSGENGRVVSHDFPKSMQSIPCMSHSSGMPLGATGL
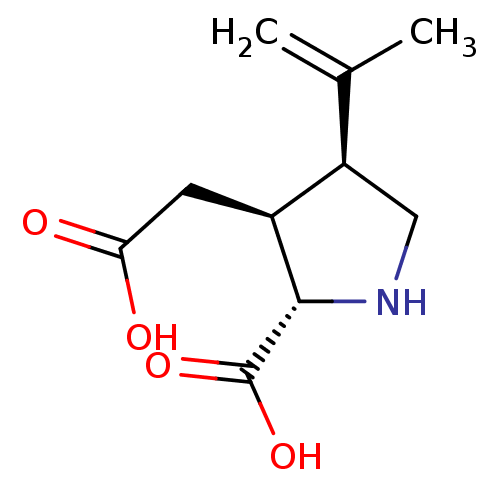
Inhibitor
Name:
BDBM50002369
Synonyms:
(2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid | (3S,4R)-3-(carboxymethyl)-4-(prop-1-en-2-yl)-L-proline | CHEMBL275040 | Digenin | Digensaeure | Helminal | Kainate | Kainsaeure | L-alpha-kainic acid | alpha-Kainic acid | digenic acid | kainic acid
Type:
Small organic molecule
Emp. Form.:
C10H15NO4
Mol. Mass.:
213.2304
SMILES:
CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r|