Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ephrin type-B receptor 3
Ligand
BDBM50332294
Substrate
n/a
Meas. Tech.
ChEMBL_774435 (CHEMBL1908652)
Kd
>10000±n/a nM
Citation
 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article More Info.:
Target
Name:
Ephrin type-B receptor 3
Synonyms:
EPHB3 | EPHB3_HUMAN | ETK2 | Ephrin receptor | Ephrin type-B receptor 3 | Ephrin type-B receptor 3 (EPHB3) | HEK2 | TYRO6 | Tyrosine Kinase EPHB3
Type:
Protein
Mol. Mass.:
110326.17
Organism:
Homo sapiens (Human)
Description:
P54753
Residue:
998
Sequence:
MARARPPPPPSPPPGLLPLLPPLLLLPLLLLPAGCRALEETLMDTKWVTSELAWTSHPESGWEEVSGYDEAMNPIRTYQVCNVRESSQNNWLRTGFIWRRDVQRVYVELKFTVRDCNSIPNIPGSCKETFNLFYYEADSDVASASSPFWMENPYVKVDTIAPDESFSRLDAGRVNTKVRSFGPLSKAGFYLAFQDQGACMSLISVRAFYKKCASTTAGFALFPETLTGAEPTSLVIAPGTCIPNAVEVSVPLKLYCNGDGEWMVPVGACTCATGHEPAAKESQCRPCPPGSYKAKQGEGPCLPCPPNSRTTSPAASICTCHNNFYRADSDSADSACTTVPSPPRGVISNVNETSLILEWSEPRDLGGRDDLLYNVICKKCHGAGGASACSRCDDNVEFVPRQLGLTERRVHISHLLAHTRYTFEVQAVNGVSGKSPLPPRYAAVNITTNQAAPSEVPTLRLHSSSGSSLTLSWAPPERPNGVILDYEMKYFEKSEGIASTVTSQMNSVQLDGLRPDARYVVQVRARTVAGYGQYSRPAEFETTSERGSGAQQLQEQLPLIVGSATAGLVFVVAVVVIAIVCLRKQRHGSDSEYTEKLQQYIAPGMKVYIDPFTYEDPNEAVREFAKEIDVSCVKIEEVIGAGEFGEVCRGRLKQPGRREVFVAIKTLKVGYTERQRRDFLSEASIMGQFDHPNIIRLEGVVTKSRPVMILTEFMENCALDSFLRLNDGQFTVIQLVGMLRGIAAGMKYLSEMNYVHRDLAARNILVNSNLVCKVSDFGLSRFLEDDPSDPTYTSSLGGKIPIRWTAPEAIAYRKFTSASDVWSYGIVMWEVMSYGERPYWDMSNQDVINAVEQDYRLPPPMDCPTALHQLMLDCWVRDRNLRPKFSQIVNTLDKLIRNAASLKVIASAQSGMSQPLLDRTVPDYTTFTTVGDWLDAIKMGRYKESFVSAGFASFDLVAQMTAEDLLRIGVTLAGHQKKILSSIQDMRLQMNQTLPVQV
Inhibitor
Name:
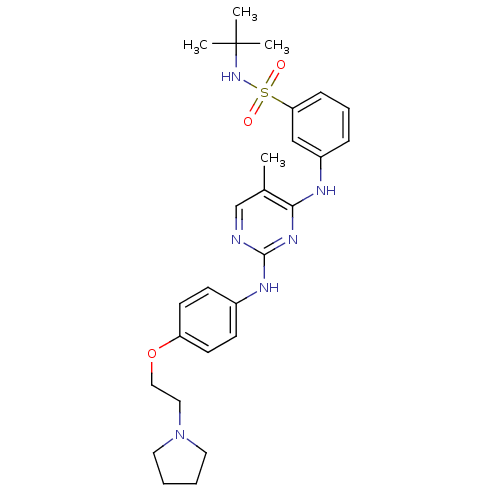
BDBM50332294
Synonyms:
CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenylamino)pyrimidin-4-ylamino)benzenesulfonamide | US10112907, Example 00018 | US10730860, TABLE 1.3 | US10766894, Compound TABLE 1.3 | US11203595, TABLE 1.3 | US11384069, Example T-5
Type:
Small organic molecule
Emp. Form.:
C27H36N6O3S
Mol. Mass.:
524.678
SMILES:
Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C