Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Indolethylamine N-methyltransferase
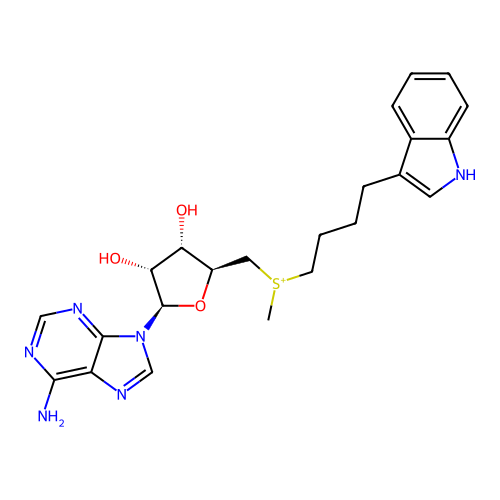
Ligand
BDBM50367125
Substrate
n/a
Meas. Tech.
ChEMBL_89037 (CHEMBL696703)
Ki
12000±n/a nM
Citation
 Benghiat, E; Crooks, PA Multisubstrate adducts as potential inhibitors of S-adenosylmethionine dependent methylases: inhibition of indole N-methyltransferase by (5'-deoxyadenosyl)[3-(3-indolyl)prop-1-yl]methylsulfonium and (5'-deoxyadenosyl)[4-(3-indolyl)but-1-yl]methylsulfonium salts. J Med Chem 26:1470-7 (1983) [PubMed] Article
Benghiat, E; Crooks, PA Multisubstrate adducts as potential inhibitors of S-adenosylmethionine dependent methylases: inhibition of indole N-methyltransferase by (5'-deoxyadenosyl)[3-(3-indolyl)prop-1-yl]methylsulfonium and (5'-deoxyadenosyl)[4-(3-indolyl)but-1-yl]methylsulfonium salts. J Med Chem 26:1470-7 (1983) [PubMed] Article More Info.:
Target
Name:
Indolethylamine N-methyltransferase
Synonyms:
Amine N-methyltransferase | Aromatic alkylamine N-methyltransferase | Arylamine N-methyltransferase | INMT | INMT_HUMAN | Indolamine N-methyltransferase
Type:
PROTEIN
Mol. Mass.:
28884.19
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1333668
Residue:
263
Sequence:
MKGGFTGGDEYQKHFLPRDYLATYYSFDGSPSPEAEMLKFNLECLHKTFGPGGLQGDTLIDIGSGPTIYQVLAACDSFQDITLSDFTDRNREELEKWLKKEPGAYDWTPAVKFACELEGNSGRWEEKEEKLRAAVKRVLKCDVHLGNPLAPAVLPLADCVLTLLAMECACCSLDAYRAALCNLASLLKPGGHLVTTVTLRLPSYMVGKREFSCVALEKEEVEQAVLDAGFDIEQLLHSPQSYSVTNAANNGVCFIVARKKPGP