Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Polyunsaturated fatty acid 5-lipoxygenase
Ligand
BDBM50006812
Substrate
n/a
Meas. Tech.
ChEMBL_4188 (CHEMBL619990)
IC50
21000±n/a nM
Citation
 Musser, JH; Kreft, AF 5-lipoxygenase: properties, pharmacology, and the quinolinyl(bridged)aryl class of inhibitors. J Med Chem 35:2501-24 (1992) [PubMed] Article
Musser, JH; Kreft, AF 5-lipoxygenase: properties, pharmacology, and the quinolinyl(bridged)aryl class of inhibitors. J Med Chem 35:2501-24 (1992) [PubMed] Article More Info.:
Target
Name:
Polyunsaturated fatty acid 5-lipoxygenase
Synonyms:
Alox5 | Arachidonate 5-lipoxygenase | LOX5_RAT
Type:
PROTEIN
Mol. Mass.:
78082.31
Organism:
Rattus norvegicus
Description:
ChEMBL_1432947
Residue:
673
Sequence:
MPSYTVTVATGSQWFAGTDDYIYLSLIGSAGCSEKHLLDKAFYNDFERGGRDSYDVTVDEELGEIYLVKIEKRKYRLHDDWYLKYITLKTPHDYIEFPCYRWITGEGEIVLRDGCAKLARDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVLNYSKAMENLFINRFMHMFQSSWHDFADFEKIFVKISNTISERVKNHWQEDLMFGYQFLNGCNPVLIKRCTELPKKLPVTTEMVECSLERQLSLEQEVQEGNIFIVDYELLDGIDANKTDPCTHQFLAAPICLLYKNLANKIVPIAIQLNQTPGEKNPIFLPTDSKYDWLLAKIWVRSSDFHIHQTITHLLRTHLVSEVFGIAMYRQLPAVHPLFKLLVAHVRFTIAINTKAREQLNCEYGLFDKANATGGGGHVQMVQRAVQDLTYSSLCFPEAIKARGMDNTEDIPYYFYRDDGLLVWEAIQSFTTEVVSIYYEDDQVVEEDQELQDFVKDVYVYGMRGRKASGFPKSIKSREKLSEYLTVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCWHLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMIRFRKNLEAIVSVIAERNKNKKLPYYYLSPDRIPNSVAI
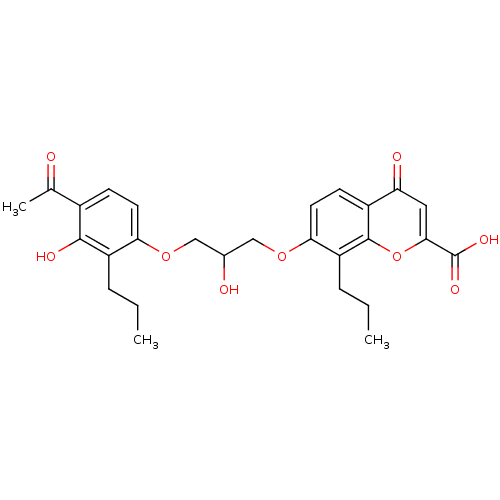
Inhibitor
Name:
BDBM50006812
Synonyms:
7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-4H-chromene-2-carboxylic acid(FPL 55,712) | 7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-8-propyl-4H-chromene-2-carboxylic acid | 7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-8-propyl-4H-chromene-2-carboxylic acid (FPL55712) | 7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-8-propyl-4H-chromene-2-carboxylic acid(FPL 55712) | 7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-8-propyl-4H-chromene-2-carboxylic acid(FPL-55712) | 7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydroxy-propoxy]-4-oxo-8-propyl-4H-chromene-2-carboxylic acid;FPL-55,712 | CHEMBL267475
Type:
Small organic molecule
Emp. Form.:
C27H30O9
Mol. Mass.:
498.5217
SMILES:
CCCc1c(OCC(O)COc2ccc3c(oc(cc3=O)C(O)=O)c2CCC)ccc(C(C)=O)c1O