Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosylhomocysteinase
Ligand
BDBM50051436
Substrate
n/a
Meas. Tech.
ChEMBL_196553 (CHEMBL801948)
Ki
43±n/a nM
Citation
 Wnuk, SF; Yuan, CS; Borchardt, RT; Balzarini, J; De Clercq, E; Robins, MJ Anticancer and antiviral effects and inactivation of S-adenosyl-L-homocysteine hydrolase with 5'-carboxaldehydes and oximes synthesized from adenosine and sugar-modified analogues. J Med Chem 40:1608-18 (1997) [PubMed] Article
Wnuk, SF; Yuan, CS; Borchardt, RT; Balzarini, J; De Clercq, E; Robins, MJ Anticancer and antiviral effects and inactivation of S-adenosyl-L-homocysteine hydrolase with 5'-carboxaldehydes and oximes synthesized from adenosine and sugar-modified analogues. J Med Chem 40:1608-18 (1997) [PubMed] Article More Info.:
Target
Name:
Adenosylhomocysteinase
Synonyms:
AHCY | Adenosylhomocysteinase | SAHH | SAHH_HUMAN
Type:
PROTEIN
Mol. Mass.:
47714.06
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1507791
Residue:
432
Sequence:
MSDKLPYKVADIGLAAWGRKALDIAENEMPGLMRMRERYSASKPLKGARIAGCLHMTVETAVLIETLVTLGAEVQWSSCNIFSTQDHAAAAIAKAGIPVYAWKGETDEEYLWCIEQTLYFKDGPLNMILDDGGDLTNLIHTKYPQLLPGIRGISEETTTGVHNLYKMMANGILKVPAINVNDSVTKSKFDNLYGCRESLIDGIKRATDVMIAGKVAVVAGYGDVGKGCAQALRGFGARVIITEIDPINALQAAMEGYEVTTMDEACQEGNIFVTTTGCIDIILGRHFEQMKDDAIVCNIGHFDVEIDVKWLNENAVEKVNIKPQVDRYRLKNGRRIILLAEGRLVNLGCAMGHPSFVMSNSFTNQVMAQIELWTHPDKYPVGVHFLPKKLDEAVAEAHLGKLNVKLTKLTEKQAQYLGMSCDGPFKPDHYRY
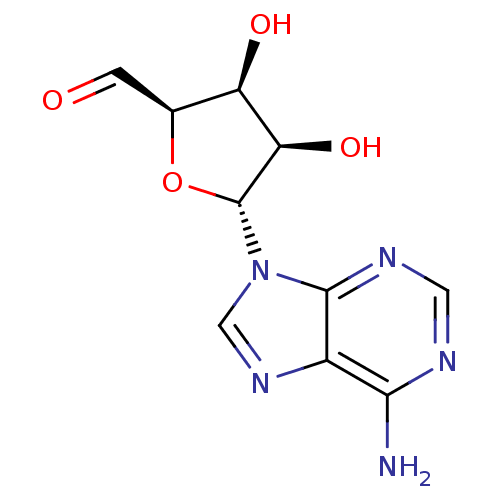
Inhibitor
Name:
BDBM50051436
Synonyms:
(2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy-tetrahydro-furan-2-carbaldehyde | 5-(6-Amino-purin-9-yl)-3,4-dihydroxy-tetrahydro-furan-2-carbaldehyde | CHEMBL77518
Type:
Small organic molecule
Emp. Form.:
C10H11N5O4
Mol. Mass.:
265.2254
SMILES:
Nc1ncnc2n(cnc12)[C@@H]1O[C@@H](C=O)[C@@H](O)[C@H]1O