Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosylhomocysteinase
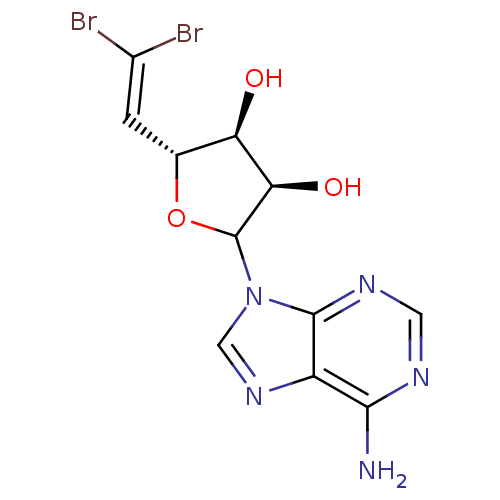
Ligand
BDBM50369380
Substrate
n/a
Meas. Tech.
ChEMBL_197352 (CHEMBL800531)
Ki
3100±n/a nM
Citation
 Wnuk, SF; Mao, Y; Yuan, CS; Borchardt, RT; Andrei, G; Balzarini, J; De Clercq, E; Robins, MJ Discovery of type II (covalent) inactivation of S-adenosyl-L-homocysteine hydrolase involving its"hydrolytic activity": synthesis and evaluation of dihalohomovinyl nucleoside analogues derived from adenosine. J Med Chem 41:3078-83 (1998) [PubMed] Article
Wnuk, SF; Mao, Y; Yuan, CS; Borchardt, RT; Andrei, G; Balzarini, J; De Clercq, E; Robins, MJ Discovery of type II (covalent) inactivation of S-adenosyl-L-homocysteine hydrolase involving its"hydrolytic activity": synthesis and evaluation of dihalohomovinyl nucleoside analogues derived from adenosine. J Med Chem 41:3078-83 (1998) [PubMed] Article More Info.:
Target
Name:
Adenosylhomocysteinase
Synonyms:
AHCY | Adenosylhomocysteinase | SAHH | SAHH_HUMAN
Type:
PROTEIN
Mol. Mass.:
47714.06
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1507791
Residue:
432
Sequence:
MSDKLPYKVADIGLAAWGRKALDIAENEMPGLMRMRERYSASKPLKGARIAGCLHMTVETAVLIETLVTLGAEVQWSSCNIFSTQDHAAAAIAKAGIPVYAWKGETDEEYLWCIEQTLYFKDGPLNMILDDGGDLTNLIHTKYPQLLPGIRGISEETTTGVHNLYKMMANGILKVPAINVNDSVTKSKFDNLYGCRESLIDGIKRATDVMIAGKVAVVAGYGDVGKGCAQALRGFGARVIITEIDPINALQAAMEGYEVTTMDEACQEGNIFVTTTGCIDIILGRHFEQMKDDAIVCNIGHFDVEIDVKWLNENAVEKVNIKPQVDRYRLKNGRRIILLAEGRLVNLGCAMGHPSFVMSNSFTNQVMAQIELWTHPDKYPVGVHFLPKKLDEAVAEAHLGKLNVKLTKLTEKQAQYLGMSCDGPFKPDHYRY