Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Integrase
Ligand
BDBM50073623
Substrate
n/a
Meas. Tech.
ChEMBL_88621 (CHEMBL701725)
IC50
1030±n/a nM
Citation
 King, PJ; Ma, G; Miao, W; Jia, Q; McDougall, BR; Reinecke, MG; Cornell, C; Kuan, J; Kim, TR; Robinson, WE Structure-activity relationships: analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. J Med Chem 42:497-509 (1999) [PubMed] Article
King, PJ; Ma, G; Miao, W; Jia, Q; McDougall, BR; Reinecke, MG; Cornell, C; Kuan, J; Kim, TR; Robinson, WE Structure-activity relationships: analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. J Med Chem 42:497-509 (1999) [PubMed] Article More Info.:
Target
Name:
Integrase
Synonyms:
Human immunodeficiency virus type 1 integrase
Type:
PROTEIN
Mol. Mass.:
32231.48
Organism:
Human immunodeficiency virus 1
Description:
ChEMBL_90865
Residue:
288
Sequence:
FLDGIDKAQDEHEKYHSNWRAMASDFNLPPVVAKEIVASCDKCQLKGEAMHGQVDCSPGIWQLDCTHLEGKVILVAVHVASGYIEAEVIPAETGQETAYFLLKLAGRWPVKTIHTDNGSNFTSTTVKAACWWAGIKQEFGIPYNPQSQGVVESMNKELKKIIGQVRDQAEHLKTAVQMAVFIHNFKRKGGIGGYSAGERIVDIIATDIQTKELQKQITKIQNFRVYYRDSRDPLWKGPAKLLWKGEGAVVIQDNSDIKVVPRRKVKIIRDYGKQMAGDDCVASRQDED
Inhibitor
Name:
BDBM50073623
Synonyms:
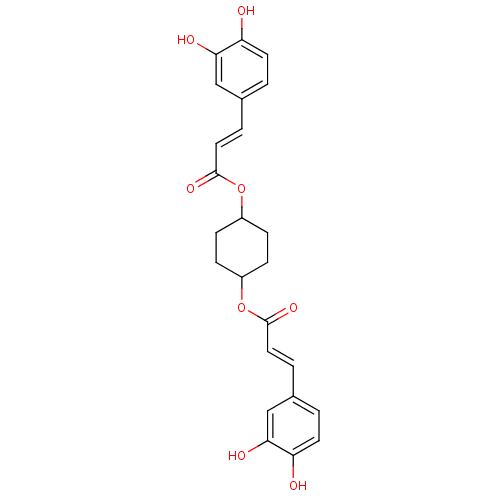
(E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid 4-[(E)-3-(3,4-dihydroxy-phenyl)-acryloyloxy]-cyclohexyl ester | CHEMBL149826
Type:
Small organic molecule
Emp. Form.:
C24H24O8
Mol. Mass.:
440.4426
SMILES:
Oc1ccc(\C=C\C(=O)OC2CCC(CC2)OC(=O)\C=C\c2ccc(O)c(O)c2)cc1O |(-2.66,-3.51,;-1.31,-4.26,;.02,-3.48,;1.36,-4.24,;1.36,-5.78,;2.7,-6.55,;4.03,-5.78,;5.38,-6.53,;5.39,-8.07,;6.71,-5.76,;8.26,-5.71,;9.06,-7.01,;10.6,-6.97,;11.34,-5.62,;10.53,-4.31,;8.99,-4.35,;12.88,-5.57,;14.22,-4.8,;14.22,-3.26,;15.56,-5.57,;16.89,-4.8,;18.22,-5.57,;18.22,-7.12,;19.56,-7.89,;20.9,-7.12,;22.24,-7.89,;20.89,-5.56,;22.22,-4.79,;19.56,-4.8,;.05,-6.57,;-1.3,-5.81,;-2.63,-6.6,)|