Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, liver form
Ligand
BDBM50222205
Substrate
n/a
Meas. Tech.
ChEMBL_321348 (CHEMBL880662)
IC50
101000±n/a nM
Citation
More Info.:
Target
Name:
Glycogen phosphorylase, liver form
Synonyms:
Glycogen phosphorylase, liver form | Lgp | Liver glycogen phosphorylase | PYGL_RAT | Pygl
Type:
PROTEIN
Mol. Mass.:
97489.46
Organism:
Rattus norvegicus
Description:
ChEMBL_1491377
Residue:
850
Sequence:
MAKPLTDQEKRRQISIRGIVGVENVAELKKGFNRHLHFTLVKDRNVATPRDYYFALAHTVRDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDMEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIREGWQVEEADDWLRHGNPWEKARPEFMLPVHFYGRVEHTQAGTKWVDTQVVLALPYDTPVPGYMNNTVNTMRLWSARAPNDFNLQDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDVIRRFKASKFGSKDGVGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKLPWSKAWEITKKTFAYTNHTVLPEALERWPVDLVEKLLPRHLQIIYEINQKHLDRIVALFPKDIDRMRRMSLIEEEGGKRINMAHLCIVGCHAVNGVAKIHSDIVKTQVFKDFSELEPDKFQNKTNGITPRRWLLLCNPGLADLIAEKIGEDYVKDLSQLTKLHSFVGDDIFLREIAKVKQENKLKFSQFLEKEYKVKINPSSMFDVHVKRIHEYKRQLLNCLHVITMYNRIKKDPKKFFVPRTVIIGGKAAPGYHMAKMIIKLVTSVAEVVNNDPMVGSKLKVIFLENYRVSLAEKVIPATDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRVDDVAALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPNQPDLFKDIINMLFYHDRFKVFADYEAYVKCQEKVSQLYMNQKAWNTMVLRNIAASGKFSSDRTIREYAKDIWNMEPSDLKISLSKESSNGVNANGK
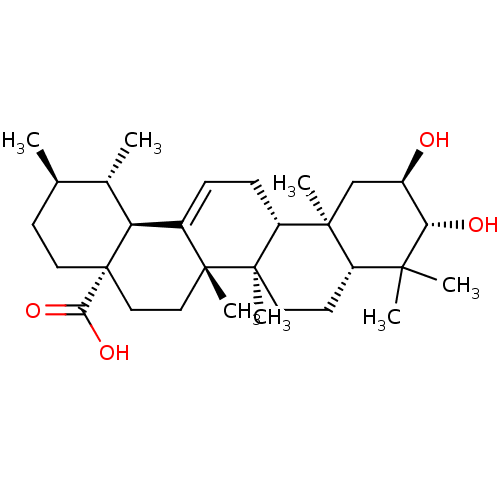
Inhibitor
Name:
BDBM50222205
Synonyms:
(1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,11-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid | 2-alpha-hydroxyursolic acid | 2alpha,2beta-dihydroxy-18beta-ursan-12-ene-28-oic acid | 2alpha,3beta-Dihydroxyurs-12-en-28-oic Acid | CHEMBL391533 | Corosolic acid | US11660306, Example Corosolic acid
Type:
Small organic molecule
Emp. Form.:
C30H48O4
Mol. Mass.:
472.6997
SMILES:
C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9|