Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Heat shock protein HSP 90-alpha
Ligand
BDBM50173904
Substrate
n/a
Meas. Tech.
ChEMBL_320742 (CHEMBL881215)
Kd
>100000±n/a nM
Citation
More Info.:
Target
Name:
Heat shock protein HSP 90-alpha
Synonyms:
HS90A_HUMAN | HSP 86 | HSP86 | HSP90A | HSP90AA1 | HSPC1 | HSPCA | Heat Shock Protein 90 (Hsp90) | Heat shock 86 kDa | Heat shock protein HSP 90 (HSP90) | Heat shock protein HSP 90-alpha (HSP90) | Heat shock protein HSP 90-alpha (HSP90A) | LAP-2 | LPS-associated protein 2 | Lipopolysaccharide-associated protein 2 | Renal carcinoma antigen NY-REN-38 | heat shock protein 90kDa alpha (cytosolic), class A member 1 isoform 2
Type:
Molecular Chaperone
Mol. Mass.:
84623.45
Organism:
Homo sapiens (Human)
Description:
P07900
Residue:
732
Sequence:
MPEETQTQDQPMEEEEVETFAFQAEIAQLMSLIINTFYSNKEIFLRELISNSSDALDKIRYESLTDPSKLDSGKELHINLIPNKQDRTLTIVDTGIGMTKADLINNLGTIAKSGTKAFMEALQAGADISMIGQFGVGFYSAYLVAEKVTVITKHNDDEQYAWESSAGGSFTVRTDTGEPMGRGTKVILHLKEDQTEYLEERRIKEIVKKHSQFIGYPITLFVEKERDKEVSDDEAEEKEDKEEEKEKEEKESEDKPEIEDVGSDEEEEKKDGDKKKKKKIKEKYIDQEELNKTKPIWTRNPDDITNEEYGEFYKSLTNDWEDHLAVKHFSVEGQLEFRALLFVPRRAPFDLFENRKKKNNIKLYVRRVFIMDNCEELIPEYLNFIRGVVDSEDLPLNISREMLQQSKILKVIRKNLVKKCLELFTELAEDKENYKKFYEQFSKNIKLGIHEDSQNRKKLSELLRYYTSASGDEMVSLKDYCTRMKENQKHIYYITGETKDQVANSAFVERLRKHGLEVIYMIEPIDEYCVQQLKEFEGKTLVSVTKEGLELPEDEEEKKKQEEKKTKFENLCKIMKDILEKKVEKVVVSNRLVTSPCCIVTSTYGWTANMERIMKAQALRDNSTMGYMAAKKHLEINPDHSIIETLRQKAEADKNDKSVKDLVILLYETALLSSGFSLEDPQTHANRIYRMIKLGLGIDEDDPTADDTSAAVTEEMPPLEGDDDTSRMEEVD
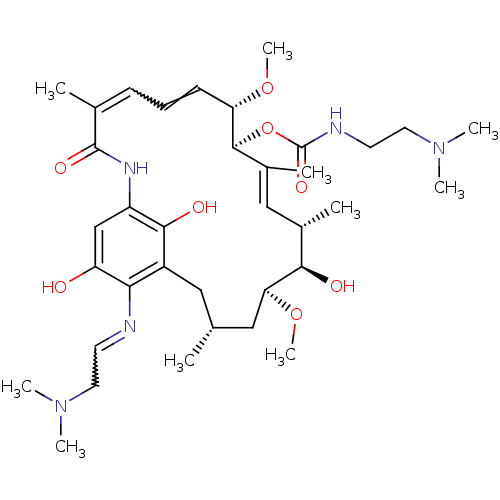
Inhibitor
Name:
BDBM50173904
Synonyms:
(2-Dimethylamino-ethyl)-carbamic acid (4E,6Z)-(13S,14S,17R,19S)-19-(2-dimethylamino-ethylamino)-13-(S)-hydroxy-8,14-dimethoxy-4,12,16-trimethyl-10-(R)-methyl-3,20,22-trioxo-2-aza-bicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl ester | CHEMBL198726
Type:
Small organic molecule
Emp. Form.:
C36H57N5O8
Mol. Mass.:
687.8665
SMILES:
CO[C@H]1C[C@H](C)Cc2c(O)c(NC(=O)C(C)=CC=C[C@H](OC)[C@@H](OC(=O)NCCN(C)C)\C(C)=C\[C@H](C)[C@H]1O)cc(O)c2N=CCN(C)C |w:16.16,44.46,t:33|