Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mitogen-activated protein kinase 8
Ligand
BDBM50181532
Substrate
n/a
Meas. Tech.
ChEMBL_330898 (CHEMBL862110)
IC50
>100000±n/a nM
Citation
 Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem 49:955-70 (2006) [PubMed] Article
Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem 49:955-70 (2006) [PubMed] Article More Info.:
Target
Name:
Mitogen-activated protein kinase 8
Synonyms:
JNK-46 | JNK1 | JNK1-alpha-1 | MAPK8 | MK08_HUMAN | Mitogen-Activated Protein Kinase 8 (JNK1) | PRKM8 | SAPK1 | SAPK1C | Stress-activated protein kinase JNK1 | c-Jun N-terminal kinase 1 | c-Jun N-terminal kinase 1 (JNK1) | c-Jun N-terminal kinase 1(JNK1) | c-Jun N-terminal kinase 2 (JNK2)
Type:
Enzyme
Mol. Mass.:
48297.57
Organism:
Homo sapiens (Human)
Description:
JNK-1 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology).
Residue:
427
Sequence:
MSRSKRDNNFYSVEIGDSTFTVLKRYQNLKPIGSGAQGIVCAAYDAILERNVAIKKLSRPFQNQTHAKRAYRELVLMKCVNHKNIIGLLNVFTPQKSLEEFQDVYIVMELMDANLCQVIQMELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTAGTSFMMTPYVVTRYYRAPEVILGMGYKENVDLWSVGCIMGEMVCHKILFPGRDYIDQWNKVIEQLGTPCPEFMKKLQPTVRTYVENRPKYAGYSFEKLFPDVLFPADSEHNKLKASQARDLLSKMLVIDASKRISVDEALQHPYINVWYDPSEAEAPPPKIPDKQLDEREHTIEEWKELIYKEVMDLEERTKNGVIRGQPSPLGAAVINGSQHPSSSSSVNDVSSMSTDPTLASDTDSSLEAAAGPLGCCR
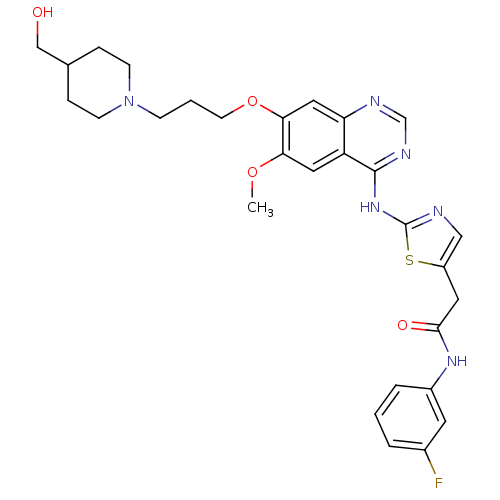
Inhibitor
Name:
BDBM50181532
Synonyms:
CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(hydroxymethyl)piperidin-1-yl)propoxy)-6-methoxyquinazolin-4-ylamino)thiazol-5-yl)acetamide | N-(3-fluorophenyl)-2-{2-[(7-{3-[4-(hydroxymethyl)piperidin-1-yl]propoxy}-6-methoxyquinazolin-4-yl)amino]-1,3-thiazol-5-yl}acetamide | N-3-fluorophenyl)-2-2-7-3-4-hydroxymethyl)piperidin-1-yl)propoxy)-6-methoxyquinazolin-4-ylamino)thiazol-5-yl)acetamide
Type:
Small organic molecule
Emp. Form.:
C29H33FN6O4S
Mol. Mass.:
580.674
SMILES:
COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1