Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ephrin type-B receptor 1
Ligand
BDBM5655
Substrate
n/a
Meas. Tech.
ChEMBL_586569 (CHEMBL1060191)
Kd
>10000±n/a nM
Citation
 Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26:127-32 (2008) [PubMed] Article
Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26:127-32 (2008) [PubMed] Article More Info.:
Target
Name:
Ephrin type-B receptor 1
Synonyms:
ELK | EPH receptor B1 | EPHB1 | EPHB1_HUMAN | EPHT2 | Ephrin receptor | Ephrin type-B receptor 1 | HEK6 | NET
Type:
Enzyme Catalytic Domain
Mol. Mass.:
109882.53
Organism:
Homo sapiens (Human)
Description:
EPH receptor B1 EPHB1 HUMAN::P54762
Residue:
984
Sequence:
MALDYLLLLLLASAVAAMEETLMDTRTATAELGWTANPASGWEEVSGYDENLNTIRTYQVCNVFEPNQNNWLLTTFINRRGAHRIYTEMRFTVRDCSSLPNVPGSCKETFNLYYYETDSVIATKKSAFWSEAPYLKVDTIAADESFSQVDFGGRLMKVNTEVRSFGPLTRNGFYLAFQDYGACMSLLSVRVFFKKCPSIVQNFAVFPETMTGAESTSLVIARGTCIPNAEEVDVPIKLYCNGDGEWMVPIGRCTCKPGYEPENSVACKACPAGTFKASQEAEGCSHCPSNSRSPAEASPICTCRTGYYRADFDPPEVACTSVPSGPRNVISIVNETSIILEWHPPRETGGRDDVTYNIICKKCRADRRSCSRCDDNVEFVPRQLGLTECRVSISSLWAHTPYTFDIQAINGVSSKSPFPPQHVSVNITTNQAAPSTVPIMHQVSATMRSITLSWPQPEQPNGIILDYEIRYYEKEHNEFNSSMARSQTNTARIDGLRPGMVYVVQVRARTVAGYGKFSGKMCFQTLTDDDYKSELREQLPLIAGSAAAGVVFVVSLVAISIVCSRKRAYSKEAVYSDKLQHYSTGRGSPGMKIYIDPFTYEDPNEAVREFAKEIDVSFVKIEEVIGAGEFGEVYKGRLKLPGKREIYVAIKTLKAGYSEKQRRDFLSEASIMGQFDHPNIIRLEGVVTKSRPVMIITEFMENGALDSFLRQNDGQFTVIQLVGMLRGIAAGMKYLAEMNYVHRDLAARNILVNSNLVCKVSDFGLSRYLQDDTSDPTYTSSLGGKIPVRWTAPEAIAYRKFTSASDVWSYGIVMWEVMSFGERPYWDMSNQDVINAIEQDYRLPPPMDCPAALHQLMLDCWQKDRNSRPRFAEIVNTLDKMIRNPASLKTVATITAVPSQPLLDRSIPDFTAFTTVDDWLSAIKMVQYRDSFLTAGFTSLQLVTQMTSEDLLRIGITLAGHQKKILNSIHSMRVQISQSPTAMA
Inhibitor
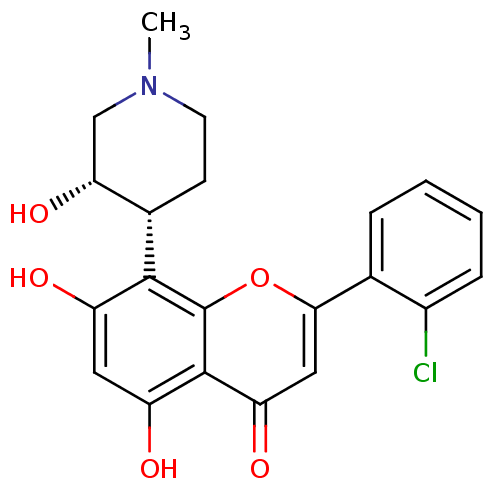
Name:
BDBM5655
Synonyms:
2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-chromen-4-one | CHEMBL428690 | Flavopiridol | US10294218, Example Flavopiridol | US9617225, Flavopiridol
Type:
Small organic molecule
Emp. Form.:
C21H20ClNO5
Mol. Mass.:
401.84
SMILES:
CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r|