Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholinesterase
Ligand
BDBM10586
Substrate
n/a
Meas. Tech.
ChEMBL_811247 (CHEMBL2015199)
IC50
44±n/a nM
Citation
 Galdeano, C; Viayna, E; Sola, I; Formosa, X; Camps, P; Badia, A; Clos, MV; Relat, J; Ratia, M; Bartolini, M; Mancini, F; Andrisano, V; Salmona, M; Minguillón, C; González-Muñoz, GC; Rodríguez-Franco, MI; Bidon-Chanal, A; Luque, FJ; Muñoz-Torrero, D Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases. J Med Chem 55:661-9 (2012) [PubMed] Article
Galdeano, C; Viayna, E; Sola, I; Formosa, X; Camps, P; Badia, A; Clos, MV; Relat, J; Ratia, M; Bartolini, M; Mancini, F; Andrisano, V; Salmona, M; Minguillón, C; González-Muñoz, GC; Rodríguez-Franco, MI; Bidon-Chanal, A; Luque, FJ; Muñoz-Torrero, D Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases. J Med Chem 55:661-9 (2012) [PubMed] Article More Info.:
Target
Name:
Cholinesterase
Synonyms:
Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase
Type:
Homotetramer
Mol. Mass.:
68422.27
Organism:
Homo sapiens (Human)
Description:
P06276
Residue:
602
Sequence:
MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDCLYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALGFLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPGSHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEILLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDVVGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLERRDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMTKLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCVGL
Inhibitor
Name:
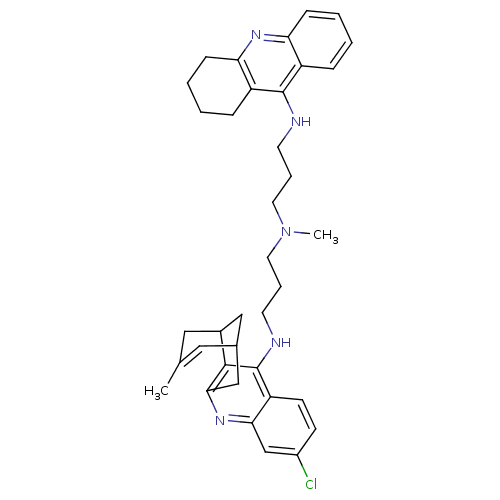
BDBM10586
Synonyms:
3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]propyl}-N-methylamino}propyl}amino}-7,11-methanocycloocta[b]quinoline trihydrochloride | CHEMBL2011487 | Huprine-Tacrine Heterodimer 17d | [3-({7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11}.0^{4,9}]heptadeca-2(11),3,5,7,9,14-hexaen-3-yl}amino)propyl](methyl)[3-(1,2,3,4-tetrahydroacridin-9-ylamino)propyl]amine trihydrochloride
Type:
Small organic molecule
Emp. Form.:
C37H44ClN5
Mol. Mass.:
594.232
SMILES:
CN(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2C3CC(Cc2nc2cc(Cl)ccc12)C=C(C)C3 |t:45|