Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nitric oxide synthase, inducible
Ligand
BDBM50148162
Substrate
n/a
Meas. Tech.
ChEMBL_854481 (CHEMBL2155040)
IC50
350±n/a nM
Citation
 Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem 58:117-27 (2012) [PubMed] Article
Stefani, HA; Gueogjan, K; Manarin, F; Farsky, SH; Zukerman-Schpector, J; Caracelli, I; Pizano Rodrigues, SR; Muscará, MN; Teixeira, SA; Santin, JR; Machado, ID; Bolonheis, SM; Curi, R; Vinolo, MA Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem 58:117-27 (2012) [PubMed] Article More Info.:
Target
Name:
Nitric oxide synthase, inducible
Synonyms:
HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS
Type:
Homodimer
Mol. Mass.:
131141.95
Organism:
Homo sapiens (Human)
Description:
P35228
Residue:
1153
Sequence:
MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPLVETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIMTPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQLTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNIRSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYGRFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVGGLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINIAVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEMLNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVTILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPGNGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGDELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDLSKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQPALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQLLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQLPILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCFVRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPDEDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLYVCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDRVAVQPSSLEMSAL
Inhibitor
Name:
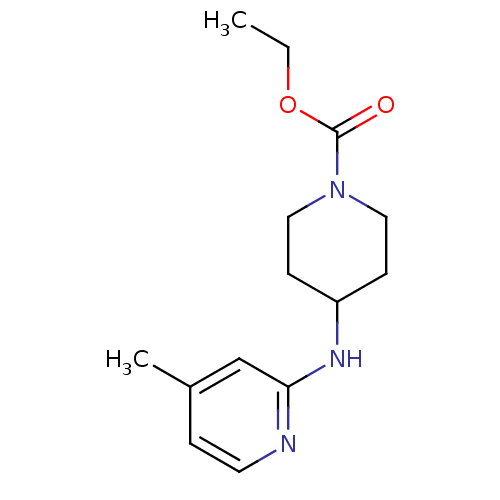
BDBM50148162
Synonyms:
4-(4-Methyl-pyridin-2-ylamino)-piperidine-1-carboxylic acid ethyl ester | CHEMBL420671 | ETHYL 4-[(4-METHYLPYRIDIN-2-YL)AMINO]PIPERIDINE-1-CARBOXYLATE | Ethyl 4-[(4-methylpyridin-2-yl)amino]piperidine-1-carboxylate, 9 | ethyl 4-(4-methylpyridin-2-ylamino)piperidine-1-carboxylate
Type:
Small organic molecule
Emp. Form.:
C14H21N3O2
Mol. Mass.:
263.3354
SMILES:
CCOC(=O)N1CCC(CC1)Nc1cc(C)ccn1