Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Voltage-dependent L-type calcium channel subunit alpha-1C
Ligand
BDBM50022815
Substrate
n/a
Meas. Tech.
ChEMBL_1479090 (CHEMBL3436066)
IC50
2000±n/a nM
Citation
 Wisniowska, B; Mendyk, A; Fijorek, K; Glinka, A; Polak, S Predictive model for L-type channel inhibition: multichannel block in QT prolongation risk assessment. J Appl Toxicol 32:858-66 (2012) [PubMed] Article
Wisniowska, B; Mendyk, A; Fijorek, K; Glinka, A; Polak, S Predictive model for L-type channel inhibition: multichannel block in QT prolongation risk assessment. J Appl Toxicol 32:858-66 (2012) [PubMed] Article More Info.:
Target
Name:
Voltage-dependent L-type calcium channel subunit alpha-1C
Synonyms:
CAC1C_CAVPO | CACH2 | CACH2 | CACN2 | CACNA1C | CACNL1A1 | CCHL1A1 | Calcium channel, L type, alpha-1 polypeptide, isoform 1, cardiac muscle | Voltage-dependent L-type calcium channel subunit alpha-1C | Voltage-gated calcium channel subunit alpha Cav1.2
Type:
PROTEIN
Mol. Mass.:
19518.62
Organism:
Cavia porcellus
Description:
ChEMBL_106600
Residue:
169
Sequence:
FQEQGEQEYKNCELDKNQRQCVEYALKARPLRRYIPISITFFRLFRVMRLVKLLSRGEGIRTLLWTFIKSFQALPYVALLIVMLFFIYAVIGMQVFGKIALNDTTEINRNNNFQTFPQAVLLLFRCATGEAWQDIMLACMPGKKRAPESEPSNSTEGETPCGSSFAVFY
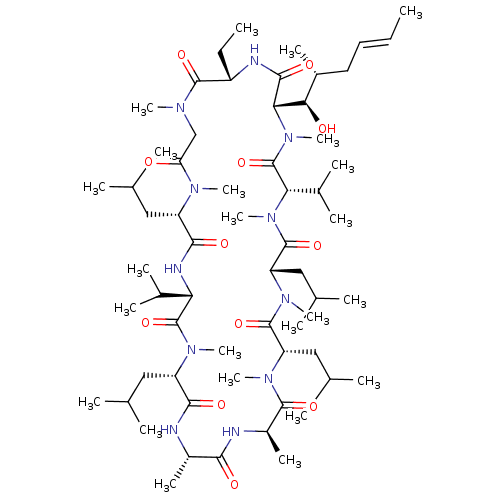
Inhibitor
Name:
BDBM50022815
Synonyms:
(3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-bis(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecone | 30-Ethyl-33-((E)-1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,28-octamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone | 30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone | CYCLOSPORINE | Cyclosporin A | Cyclosporine A | Cyclosproine A | US10077289, Compound Cyclosporin A | US9138393, Cyclosporin A
Type:
Small organic molecule
Emp. Form.:
C62H111N11O12
Mol. Mass.:
1202.6112
SMILES:
CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r|