Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sodium/potassium-transporting ATPase subunit alpha-1
Ligand
BDBM50096257
Substrate
n/a
Meas. Tech.
ChEMBL_1506103 (CHEMBL3595969)
IC50
11000±n/a nM
Citation
 Alves, SL; Paixão, N; Ferreira, LG; Santos, FR; Neves, LD; Oliveira, GC; Cortes, VF; Salomé, KS; Barison, A; Santos, FV; Cenzi, G; Varotti, FP; Oliveira, SM; Taranto, AG; Comar, M; Silva, LM; Noël, F; Quintas, LE; Barbosa, LA; Villar, JA ¿-Benzylidene digoxin derivatives synthesis and molecular modeling: Evaluation of anticancer and the Na,K-ATPase activity effect. Bioorg Med Chem 23:4397-404 (2015) [PubMed] Article
Alves, SL; Paixão, N; Ferreira, LG; Santos, FR; Neves, LD; Oliveira, GC; Cortes, VF; Salomé, KS; Barison, A; Santos, FV; Cenzi, G; Varotti, FP; Oliveira, SM; Taranto, AG; Comar, M; Silva, LM; Noël, F; Quintas, LE; Barbosa, LA; Villar, JA ¿-Benzylidene digoxin derivatives synthesis and molecular modeling: Evaluation of anticancer and the Na,K-ATPase activity effect. Bioorg Med Chem 23:4397-404 (2015) [PubMed] Article More Info.:
Target
Name:
Sodium/potassium-transporting ATPase subunit alpha-1
Synonyms:
AT1A1_HUMAN | ATP1A1 | Na(+)/K(+) ATPase alpha-1 subunit | Na,K-ATPase isoform alpha-1 (α1) | Sodium pump subunit alpha-1 | Sodium/potassium-transporting ATPase subunit alpha-1
Type:
Enzyme
Mol. Mass.:
112879.20
Organism:
Homo sapiens (Human)
Description:
P05023
Residue:
1023
Sequence:
MGKGVGRDKYEPAAVSEQGDKKGKKGKKDRDMDELKKEVSMDDHKLSLDELHRKYGTDLSRGLTSARAAEILARDGPNALTPPPTTPEWIKFCRQLFGGFSMLLWIGAILCFLAYSIQAATEEEPQNDNLYLGVVLSAVVIITGCFSYYQEAKSSKIMESFKNMVPQQALVIRNGEKMSINAEEVVVGDLVEVKGGDRIPADLRIISANGCKVDNSSLTGESEPQTRSPDFTNENPLETRNIAFFSTNCVEGTARGIVVYTGDRTVMGRIATLASGLEGGQTPIAAEIEHFIHIITGVAVFLGVSFFILSLILEYTWLEAVIFLIGIIVANVPEGLLATVTVCLTLTAKRMARKNCLVKNLEAVETLGSTSTICSDKTGTLTQNRMTVAHMWFDNQIHEADTTENQSGVSFDKTSATWLALSRIAGLCNRAVFQANQENLPILKRAVAGDASESALLKCIELCCGSVKEMRERYAKIVEIPFNSTNKYQLSIHKNPNTSEPQHLLVMKGAPERILDRCSSILLHGKEQPLDEELKDAFQNAYLELGGLGERVLGFCHLFLPDEQFPEGFQFDTDDVNFPIDNLCFVGLISMIDPPRAAVPDAVGKCRSAGIKVIMVTGDHPITAKAIAKGVGIISEGNETVEDIAARLNIPVSQVNPRDAKACVVHGSDLKDMTSEQLDDILKYHTEIVFARTSPQQKLIIVEGCQRQGAIVAVTGDGVNDSPALKKADIGVAMGIAGSDVSKQAADMILLDDNFASIVTGVEEGRLIFDNLKKSIAYTLTSNIPEITPFLIFIIANIPLPLGTVTILCIDLGTDMVPAISLAYEQAESDIMKRQPRNPKTDKLVNERLISMAYGQIGMIQALGGFFTYFVILAENGFLPIHLLGLRVDWDDRWINDVEDSYGQQWTYEQRKIVEFTCHTAFFVSIVVVQWADLVICKTRRNSVFQQGMKNKILIFGLFEETALAAFLSYCPGMGVALRMYPLKPTWWFCAFPYSLLIFVYDEVRKLIIRRRPGGWVEKETYY
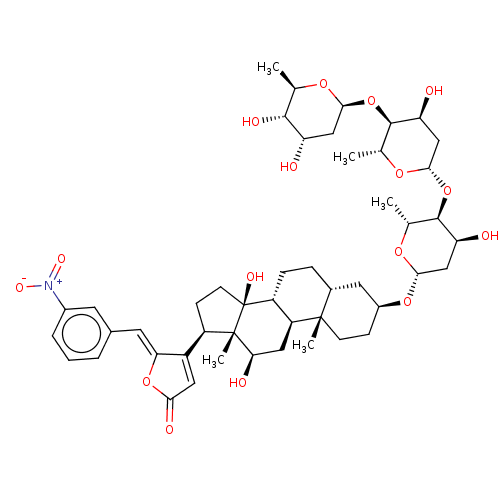
Inhibitor
Name:
BDBM50096257
Synonyms:
CHEMBL3594280
Type:
Small organic molecule
Emp. Form.:
C48H67NO16
Mol. Mass.:
914.0427
SMILES:
[H][C@@]1(C[C@H](O)[C@H](O[C@@]2([H])C[C@H](O)[C@H](O[C@@]3([H])C[C@H](O)[C@H](O)[C@@H](C)O3)[C@@H](C)O2)[C@@H](C)O1)O[C@H]1CC[C@@]2(C)[C@]([H])(CC[C@]3([H])[C@]2([H])C[C@@H](O)[C@]2(C)[C@H](CC[C@]32O)C2=CC(=O)O\C2=C/c2cccc(c2)[N+]([O-])=O)C1 |r,t:60|