Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
RAC-alpha serine/threonine-protein kinase
Ligand
BDBM2579
Substrate
n/a
Meas. Tech.
ChEMBL_1577735 (CHEMBL3807613)
IC50
18±n/a nM
Citation
More Info.:
Target
Name:
RAC-alpha serine/threonine-protein kinase
Synonyms:
AKT phosphorylation (p-AKT) | AKT1 | AKT1/PPP1CA | AKT1_HUMAN | C-AKT | PKB | PKB alpha | Protein kinase Akt-1 | Protein kinase B | Protein kinase B (AKT1) | Protein kinase B (Akt 1) | Protein kinase B (Akt) | Protein kinase B alpha | Protein kinase B alpha (AKT1) | Proto-oncogene Akt (Akt1) | Proto-oncogene c-Akt (AKT) | Proto-oncogene c-Akt (AKT1) | RAC | RAC-PK-alpha | RAC-alpha serine/threonine-protein kinase (AKT) | RAC-alpha serine/threonine-protein kinase (AKT1) | RAC-alpha serine/threonine-protein kinase (pAKT)
Type:
Enzyme
Mol. Mass.:
55681.25
Organism:
Homo sapiens (Human)
Description:
P31749
Residue:
480
Sequence:
MSDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQREAPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWTTAIQTVADGLKKQEEEEMDFRSGSPSDNSGAEEMEVSLAKPKHRVTMNEFEYLKLLGKGTFGKVILVKEKATGRYYAMKILKKEVIVAKDEVAHTLTENRVLQNSRHPFLTALKYSFQTHDRLCFVMEYANGGELFFHLSRERVFSEDRARFYGAEIVSALDYLHSEKNVVYRDLKLENLMLDKDGHIKITDFGLCKEGIKDGATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILMEEIRFPRTLGPEAKSLLSGLLKKDPKQRLGGGSEDAKEIMQHRFFAGIVWQHVYEKKLSPPFKPQVTSETDTRYFDEEFTAQMITITPPDQDDSMECVDSERRPHFPQFSYSASGTA
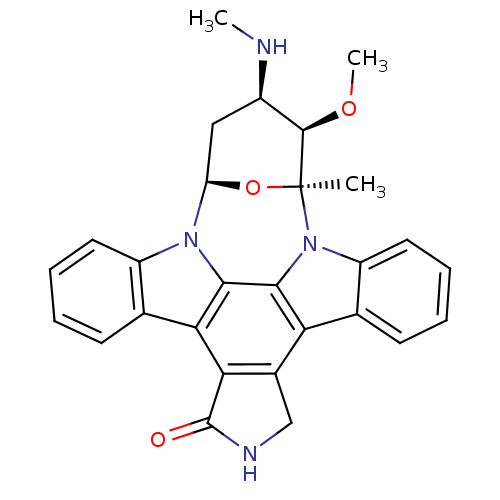
Inhibitor
Name:
BDBM2579
Synonyms:
(2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8(13),9,11,14(28),15(19),20(27),21(26),22,24-nonaen-16-one | CHEMBL388978 | Staurosporin, 4 | Staurosporine | Staurosporine, 8 | US20240002365, Compound staurosporine | US9206188, Staurosporine | US9226923, Staurosporine
Type:
Small organic molecule
Emp. Form.:
C28H26N4O3
Mol. Mass.:
466.531
SMILES:
CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r|